Genetically encoded lipid-polypeptide hybrid biomaterials that exhibit temperature-triggered hierarchical self-assembly
- PMID: 29556049
- PMCID: PMC6676901
- DOI: 10.1038/s41557-018-0005-z
Genetically encoded lipid-polypeptide hybrid biomaterials that exhibit temperature-triggered hierarchical self-assembly
Abstract
Post-translational modification of proteins is a strategy widely used in biological systems. It expands the diversity of the proteome and allows for tailoring of both the function and localization of proteins within cells as well as the material properties of structural proteins and matrices. Despite their ubiquity in biology, with a few exceptions, the potential of post-translational modifications in biomaterials synthesis has remained largely untapped. As a proof of concept to demonstrate the feasibility of creating a genetically encoded biohybrid material through post-translational modification, we report here the generation of a family of three stimulus-responsive hybrid materials-fatty-acid-modified elastin-like polypeptides-using a one-pot recombinant expression and post-translational lipidation methodology. These hybrid biomaterials contain an amphiphilic domain, composed of a β-sheet-forming peptide that is post-translationally functionalized with a C14 alkyl chain, fused to a thermally responsive elastin-like polypeptide. They exhibit temperature-triggered hierarchical self-assembly across multiple length scales with varied structure and material properties that can be controlled at the sequence level.
Figures




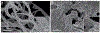
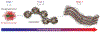
References
-
- Langer R & Tirrell DA Designing materials for biology and medicine. Nature 428, 487–492 (2004). - PubMed
-
- Maskarinec SA & Tirrell DA Protein engineering approaches to biomaterials design. Curr. Opin. Biotechnol. 16, 422–426 (2005). - PubMed
-
- Chilkoti A, Dreher MR & Meyer DE Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv. Drug Deliv. Rev. 54, 1093–1111 (2002). - PubMed
-
- Haider M, Megeed Z & Ghandehari H Genetically engineered polymers: Status and prospects for controlled release. J. Control. Release 95, 1–26 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

