Self-assembling asymmetric peptide-dendrimer micelles - a platform for effective and versatile in vitro nucleic acid delivery
- PMID: 29556057
- PMCID: PMC5859181
- DOI: 10.1038/s41598-018-22902-9
Self-assembling asymmetric peptide-dendrimer micelles - a platform for effective and versatile in vitro nucleic acid delivery
Abstract
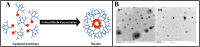
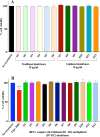
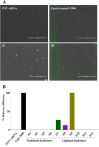
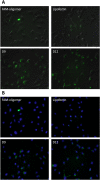
Despite advancements in the development of high generation cationic-dendrimer systems for delivery of nucleic acid-based therapeutics, commercially available chemical agents suffer from major drawbacks such as cytotoxicity while being laborious and costly to synthesize. To overcome the aforementioned limitations, low-generation cationic peptide asymmetric dendrimers with side arm lipid (cholic and decanoic acid) conjugation were designed, synthesized and systematically screened for their ability to self-assemble into micelles using dynamic light scattering. Cytotoxicity profiling revealed that our entire asymmetric peptide dendrimer library when trialled alone, or as asymmetric dendrimer micelle-nucleic acid complexes, were non-cytotoxic across a broad concentration range. Further, the delivery efficiency of asymmetric peptide dendrimers in H-4-II-E (rat hepatoma), H2K (mdx mouse myoblast), and DAOY (human medulloblastoma) cells demonstrated that cholic acid-conjugated asymmetric dendrimers possess far superior delivery efficiency when compared to the commercial standards, Lipofectamine 2000 or Lipofectin®.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
RGD peptide-modified multifunctional dendrimer platform for drug encapsulation and targeted inhibition of cancer cells.Colloids Surf B Biointerfaces. 2015 Jan 1;125:82-9. doi: 10.1016/j.colsurfb.2014.11.004. Epub 2014 Nov 11. Colloids Surf B Biointerfaces. 2015. PMID: 25437067
-
Self-Assembling Supramolecular Dendrimers for Biomedical Applications: Lessons Learned from Poly(amidoamine) Dendrimers.Acc Chem Res. 2020 Dec 15;53(12):2936-2949. doi: 10.1021/acs.accounts.0c00589. Epub 2020 Dec 4. Acc Chem Res. 2020. PMID: 33275845
-
A Dual Targeting Dendrimer-Mediated siRNA Delivery System for Effective Gene Silencing in Cancer Therapy.J Am Chem Soc. 2018 Nov 28;140(47):16264-16274. doi: 10.1021/jacs.8b10021. Epub 2018 Nov 1. J Am Chem Soc. 2018. PMID: 30346764
-
Dendrimer nanocarriers as versatile vectors in gene delivery.Nanomedicine. 2010 Feb;6(1):25-34. doi: 10.1016/j.nano.2009.05.005. Epub 2009 May 18. Nanomedicine. 2010. Retraction in: Nanomedicine. 2010 Dec;6(6):815. doi: 10.1016/j.nano.2010.11.001. PMID: 19450708 Retracted. Review.
-
Cationic Dendrimers for siRNA Delivery: An Overview of Methods for In Vitro/In Vivo Characterization.Methods Mol Biol. 2021;2282:209-244. doi: 10.1007/978-1-0716-1298-9_14. Methods Mol Biol. 2021. PMID: 33928579 Review.
Cited by
-
Medulloblastoma: Molecular Targets and Innovative Theranostic Approaches.Pharmaceutics. 2025 Jun 4;17(6):736. doi: 10.3390/pharmaceutics17060736. Pharmaceutics. 2025. PMID: 40574048 Free PMC article. Review.
-
Polypeptide-Based Systems: From Synthesis to Application in Drug Delivery.Pharmaceutics. 2023 Nov 20;15(11):2641. doi: 10.3390/pharmaceutics15112641. Pharmaceutics. 2023. PMID: 38004619 Free PMC article. Review.
-
Polymeric-Micelle-Based Delivery Systems for Nucleic Acids.Pharmaceutics. 2023 Jul 26;15(8):2021. doi: 10.3390/pharmaceutics15082021. Pharmaceutics. 2023. PMID: 37631235 Free PMC article. Review.
-
EGDMA- and TRIM-Based Microparticles Imprinted with 5-Fluorouracil for Prolonged Drug Delivery.Polymers (Basel). 2022 Mar 4;14(5):1027. doi: 10.3390/polym14051027. Polymers (Basel). 2022. PMID: 35267850 Free PMC article.
-
Ionizable drug delivery systems for efficient and selective gene therapy.Mil Med Res. 2023 Feb 27;10(1):9. doi: 10.1186/s40779-023-00445-z. Mil Med Res. 2023. PMID: 36843103 Free PMC article. Review.
References
-
- Tupally, K. R., Kokil, G. R., Thakur, S. S., Singh, P. & Parekh, H. S. in Controlled Release Systems: Advances in Nanobottles and active Nanoparticles (eds J Forcada, A van Herk, & G Pastorin) (Pan Stanford 2015).
-
- Janaszewska A, et al. Cytotoxicity of PAMAM, PPI and maltose modified PPI dendrimers in Chinese hamster ovary (CHO) and human ovarian carcinoma (SKOV3) cells. New Journal of Chemistry. 2012;36:428–437. doi: 10.1039/C1NJ20489K. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

