ILF2 and ILF3 are autoantigens in canine systemic autoimmune disease
- PMID: 29556082
- PMCID: PMC5859008
- DOI: 10.1038/s41598-018-23034-w
ILF2 and ILF3 are autoantigens in canine systemic autoimmune disease
Abstract
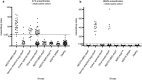
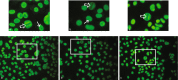
Dogs can spontaneously develop complex systemic autoimmune disorders, with similarities to human autoimmune disease. Autoantibodies directed at self-antigens are a key feature of these autoimmune diseases. Here we report the identification of interleukin enhancer-binding factors 2 and 3 (ILF2 and ILF3) as autoantigens in canine immune-mediated rheumatic disease. The ILF2 autoantibodies were discovered in a small, selected canine cohort through the use of human protein arrays; a method not previously described in dogs. Subsequently, ILF3 autoantibodies were also identified in the same cohort. The results were validated with an independent method in a larger cohort of dogs. ILF2 and ILF3 autoantibodies were found exclusively, and at a high frequency, in dogs that showed a speckled pattern of antinuclear antibodies on immunofluorescence. ILF2 and ILF3 autoantibodies were also found at low frequency in human patients with SLE and Sjögren's syndrome. These autoantibodies have the potential to be used as diagnostic biomarkers for canine, and possibly also human, autoimmune disease.
Conflict of interest statement
Stefanie Renneker and Erik Lattwein are employees of EUROIMMUN which supplied and performed a major part of the IIF-ANA analyses. Olle Kämpe is a board member of Olink Biosciences AB.
Figures



Similar articles
-
Origin and structure of autoantibodies and antigens in autoimmune rheumatic diseases.Lupus. 2008 Mar;17(3):232-5. doi: 10.1177/0961203307088246. Lupus. 2008. PMID: 18372367 No abstract available.
-
Identification of cyclin A as a molecular target of antinuclear antibodies (ANA) in hepatic and non-hepatic autoimmune diseases.J Hepatol. 1996 Dec;25(6):859-66. doi: 10.1016/s0168-8278(96)80290-x. J Hepatol. 1996. PMID: 9007714
-
The interaction between anti-Ro/SSA and anti-La/SSB autoantibodies and anti-infectious antibodies in a wide spectrum of auto-immune diseases: another angle of the autoimmune mosaic.Clin Exp Rheumatol. 2017 Nov-Dec;35(6):929-935. Epub 2017 Jul 6. Clin Exp Rheumatol. 2017. PMID: 28770708
-
Pathophysiology of antinuclear antibodies in systemic lupus erythematosus and related diseases.Adv Dent Res. 1996 Apr;10(1):44-6. doi: 10.1177/08959374960100010801. Adv Dent Res. 1996. PMID: 8934923 Review.
-
Antinuclear antibodies: diagnostic markers and clues to the basis of systemic autoimmunity.Pediatr Infect Dis J. 1988 May;7(5 Suppl):S3-9. Pediatr Infect Dis J. 1988. PMID: 2456507 Review.
Cited by
-
A scoping review of autoantibodies as biomarkers for canine autoimmune disease.J Vet Intern Med. 2022 Mar;36(2):363-378. doi: 10.1111/jvim.16392. Epub 2022 Feb 22. J Vet Intern Med. 2022. PMID: 35192227 Free PMC article.
-
ILF2: a multifaceted regulator in malignant tumors and its prospects as a biomarker and therapeutic target.Front Oncol. 2024 Dec 13;14:1513979. doi: 10.3389/fonc.2024.1513979. eCollection 2024. Front Oncol. 2024. PMID: 39735599 Free PMC article. Review.
-
An autoantigen profile of human A549 lung cells reveals viral and host etiologic molecular attributes of autoimmunity in COVID-19.J Autoimmun. 2021 Jun;120:102644. doi: 10.1016/j.jaut.2021.102644. Epub 2021 Apr 27. J Autoimmun. 2021. PMID: 33971585 Free PMC article.
-
A Master Autoantigen-ome Links Alternative Splicing, Female Predilection, and COVID-19 to Autoimmune Diseases.bioRxiv [Preprint]. 2021 Aug 4:2021.07.30.454526. doi: 10.1101/2021.07.30.454526. bioRxiv. 2021. Update in: J Transl Autoimmun. 2022;5:100147. doi: 10.1016/j.jtauto.2022.100147. PMID: 34373855 Free PMC article. Updated. Preprint.
-
An Autoantigen Profile of Human A549 Lung Cells Reveals Viral and Host Etiologic Molecular Attributes of Autoimmunity in COVID-19.bioRxiv [Preprint]. 2021 Feb 22:2021.02.21.432171. doi: 10.1101/2021.02.21.432171. bioRxiv. 2021. Update in: J Autoimmun. 2021 Jun;120:102644. doi: 10.1016/j.jaut.2021.102644. PMID: 33655248 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

