IGF-1 receptor cleavage in hypertension
- PMID: 29556095
- PMCID: PMC8075889
- DOI: 10.1038/s41440-018-0023-7
IGF-1 receptor cleavage in hypertension
Abstract
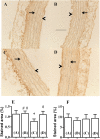
Increased protease activity causes receptor dysfunction due to extracellular cleavage of different membrane receptors in hypertension. The vasodilatory effects of insulin-like growth factor-1 (IGF-1) are decreased in hypertension. Therefore, in the present study the association of an enhanced protease activity and IGF-1 receptor cleavage was investigated using the spontaneously hypertensive rats (SHRs) and their normotensive Wistar Kyoto (WKY) controls (n = 4). Matrix metalloproteinase (MMP) activities were determined using gelatin zymography on plasma and different tissue samples. WKY aorta rings were incubated in WKY or SHR plasma with or without MMP inhibitors, and immunohistochemistry was used to quantify the densities of the alpha and beta IGF-1 receptor (IGF-1R) subunits and to determine receptor cleavage. The pAkt and peNOS levels in the aorta were investigated using immunoblotting as a measure of IGF-IR function. Increased MMP-2 and MMP-9 activities were detected in plasma and peripheral tissues of SHRs. IGF-1R beta labeling was similar in both groups without plasma incubation, but the fraction of immunolabeled area for IGF-1R alpha was lower in the endothelial layer of the SHR aorta (p < 0.05). A 24-h incubation of WKY aorta with SHR plasma did not affect the IGF-1R beta labeling density, but reduced the IGF-1R alpha labeling density in the endothelium (p < 0.05). MMP inhibitors prevented this decrease (p < 0.01). Western blot analyses revealed that the pAkt and peNOS levels under IGF-1-stimulated and -unstimulated conditions were lower in SHRs (p < 0.05). A reduced IGF-1 cellular response in the aorta was associated with the decrease in the IGF-1R alpha subunit in the SHR hypertension model. Our results indicate that MMP-dependent receptor cleavage contributed to the reduced IGF-1 response in SHRs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats.Am J Physiol Heart Circ Physiol. 2010 Jul;299(1):H25-35. doi: 10.1152/ajpheart.00620.2009. Epub 2010 Apr 9. Am J Physiol Heart Circ Physiol. 2010. PMID: 20382857 Free PMC article.
-
Cleavage and reduced CD36 ectodomain density on heart and spleen macrophages in the spontaneously hypertensive rat.Microvasc Res. 2014 Sep;95:131-42. doi: 10.1016/j.mvr.2014.08.004. Epub 2014 Aug 27. Microvasc Res. 2014. PMID: 25172177 Free PMC article.
-
Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: effects of endothelin receptor blockade.J Hypertens. 2005 Sep;23(9):1717-24. doi: 10.1097/01.hjh.0000176787.04753.ee. J Hypertens. 2005. PMID: 16093917
-
Nuclear factor kappa B and matrix metalloproteinase induced receptor cleavage in the spontaneously hypertensive rat.Hypertension. 2011 Feb;57(2):261-8. doi: 10.1161/HYPERTENSIONAHA.110.158709. Epub 2011 Jan 10. Hypertension. 2011. PMID: 21220710 Free PMC article.
-
Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneously hypertensive rat.Hypertension. 2008 Aug;52(2):415-23. doi: 10.1161/HYPERTENSIONAHA.107.104356. Epub 2008 Jul 7. Hypertension. 2008. PMID: 18606910 Free PMC article.
Cited by
-
Matrisome-Associated Gene Expression Patterns Correlating with TIMP2 in Cancer.Sci Rep. 2019 Dec 27;9(1):20142. doi: 10.1038/s41598-019-56632-3. Sci Rep. 2019. PMID: 31882975 Free PMC article.
-
Serum insulin-like growth factor-1 as a potential prognostic biomarker for heart failure with reduced ejection fraction: a meta-analysis.Front Cardiovasc Med. 2024 Sep 17;11:1415238. doi: 10.3389/fcvm.2024.1415238. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39355348 Free PMC article.
-
Pancreatic source of protease activity in the spontaneously hypertensive rat and its reduction during temporary food restriction.Microcirculation. 2019 Aug;26(6):e12548. doi: 10.1111/micc.12548. Epub 2019 May 3. Microcirculation. 2019. PMID: 30946505 Free PMC article.
-
Molecular subtyping of hypertensive disorders of pregnancy.Nat Commun. 2025 Apr 8;16(1):2948. doi: 10.1038/s41467-025-58157-y. Nat Commun. 2025. PMID: 40199872 Free PMC article.
-
New insights on the cardiovascular effects of IGF-1.Front Endocrinol (Lausanne). 2023 Feb 9;14:1142644. doi: 10.3389/fendo.2023.1142644. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36843588 Free PMC article.
References
-
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

