PLAC1-specific TCR-engineered T cells mediate antigen-specific antitumor effects in breast cancer
- PMID: 29556312
- PMCID: PMC5844056
- DOI: 10.3892/ol.2018.8075
PLAC1-specific TCR-engineered T cells mediate antigen-specific antitumor effects in breast cancer
Abstract
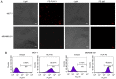
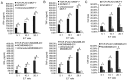
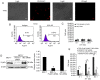
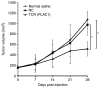
Placenta-specific 1 (PLAC1), a novel cancer-testis antigen (CTA), is expressed in a number of different human malignancies. It is frequently produced in breast cancer, serving a function in tumorigenesis. Adoptive immunotherapy using T cell receptor (TCR)-engineered T cells against CTA mediates objective tumor regression; however, to the best of our knowledge, targeting PLAC1 using engineered T cells has not yet been attempted. In the present study, the cDNAs encoding TCRα- and β-chains specific for human leukocyte antigen (HLA)-A*0201-restricted PLAC1 were cloned from a cytotoxic T-lymphocyte, generated by in vitro by the stimulation of CD8+ T cells using autologous HLA-A2+ dendritic cells loaded with a PLAC1-specific peptide (p28-36, VLCSIDWFM). The TCRα/β-chains were linked by a 2A peptide linker (TCRα-Thosea asigna virus-TCRβ), and the constructs were cloned into the lentiviral vector, followed by transduction into human cytotoxic (CD8+) T cells. The efficiency of transduction was up to 25.16%, as detected by PLAC1 multimers. TCR-transduced CD8+ T cells, co-cultured with human non-metastatic breast cancer MCF-7 cells (PLAC1+, HLA-A2+) and triple-negative breast cancer MDAMB-231 cells (PLAC1+, HLA-A2+), produced interferon γ and tumor necrosis factor α, suggesting TCR activation. Furthermore, the PLAC1 TCR-transduced CD8+ T cells efficiently and specifically identified and annihilated the HLA-A2+/PLAC1+ breast cancer cell lines in a lactate dehydrogenase activity assay. Western blot analysis demonstrated that TCR transduction stimulated the production of mitogen-activated protein kinase signaling molecules, extracellular signal-regulated kinases 1/2 and nuclear factor-κB, through phosphoinositide 3-kinase γ-mediated phosphorylation of protein kinase B in CD8+ T cells. Xenograft mouse assays revealed that PLAC1 TCR-transduced CD8+T cells significantly delayed the tumor progression in mice-bearing breast cancer compared with normal saline or negative control-transduced groups. In conclusion, a novel HLA-A2-restricted and PLAC1-specific TCR was identified. The present study demonstrated PLAC1 to be a potential target for breast cancer treatment; and the usage of PLAC1-specific TCR-engineered T cells may be a novel strategy for PLAC1-positive breast cancer treatment.
Keywords: T cell receptor; breast cancer; cancer immunotherapy; cytotoxic T cells; placenta specific 1.
Figures


Similar articles
-
Identification of a novel HLA-A2-restricted cytotoxic T lymphocyte epitope from cancer-testis antigen PLAC1 in breast cancer.Amino Acids. 2012 Jun;42(6):2257-65. doi: 10.1007/s00726-011-0966-3. Epub 2011 Jun 28. Amino Acids. 2012. PMID: 21710262
-
Avidity characterization of genetically engineered T-cells with novel and established approaches.BMC Immunol. 2016 Jul 13;17(1):23. doi: 10.1186/s12865-016-0162-z. BMC Immunol. 2016. PMID: 27411667 Free PMC article.
-
Identification of two new HLA-A*0201-restricted cytotoxic T lymphocyte epitopes from colorectal carcinoma-associated antigen PLAC1/CP1.J Gastroenterol. 2014 Mar;49(3):419-26. doi: 10.1007/s00535-013-0811-4. Epub 2013 Apr 21. J Gastroenterol. 2014. PMID: 23604623
-
Antitumor cytotoxic T-lymphocyte response in human lung carcinoma: identification of a tumor-associated antigen.Immunol Rev. 2002 Oct;188:114-21. doi: 10.1034/j.1600-065x.2002.18810.x. Immunol Rev. 2002. PMID: 12445285 Review.
-
WT1-specific T cell receptor gene therapy: improving TCR function in transduced T cells.Blood Cells Mol Dis. 2008 Jan-Feb;40(1):113-6. doi: 10.1016/j.bcmd.2007.06.018. Epub 2007 Sep 12. Blood Cells Mol Dis. 2008. PMID: 17855129 Review.
Cited by
-
Investigation of Expression Profile of Placenta-specific 1 (PLAC1) in Acute Myeloid and Lymphoid Leukemias.Avicenna J Med Biotechnol. 2023 Jul-Sep;15(3):167-172. doi: 10.18502/ajmb.v15i3.12926. Avicenna J Med Biotechnol. 2023. PMID: 37538244 Free PMC article.
-
Strategies to Overcome Failures in T-Cell Immunotherapies by Targeting PI3K-δ and -γ.Front Immunol. 2021 Aug 26;12:718621. doi: 10.3389/fimmu.2021.718621. eCollection 2021. Front Immunol. 2021. PMID: 34512641 Free PMC article. Review.
-
Protective Effect and Mechanism of Placenta Extract on Liver.Nutrients. 2022 Nov 29;14(23):5071. doi: 10.3390/nu14235071. Nutrients. 2022. PMID: 36501102 Free PMC article. Review.
-
Immunotherapy and immunoengineering for breast cancer; a comprehensive insight into CAR-T cell therapy advancements, challenges and prospects.Cell Oncol (Dordr). 2022 Oct;45(5):755-777. doi: 10.1007/s13402-022-00700-w. Epub 2022 Aug 9. Cell Oncol (Dordr). 2022. PMID: 35943716 Review.
-
PLAC1: biology and potential application in cancer immunotherapy.Cancer Immunol Immunother. 2019 Jul;68(7):1039-1058. doi: 10.1007/s00262-019-02350-8. Epub 2019 Jun 5. Cancer Immunol Immunother. 2019. PMID: 31165204 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
