Dual-Energy CT Imaging of Tumor Liposome Delivery After Gold Nanoparticle-Augmented Radiation Therapy
- PMID: 29556356
- PMCID: PMC5858500
- DOI: 10.7150/thno.22621
Dual-Energy CT Imaging of Tumor Liposome Delivery After Gold Nanoparticle-Augmented Radiation Therapy
Abstract
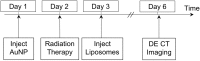
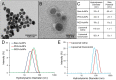
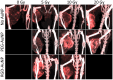
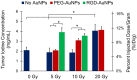
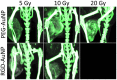
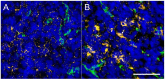
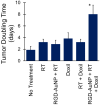
Gold nanoparticles (AuNPs) are emerging as promising agents for both cancer therapy and computed tomography (CT) imaging. AuNPs absorb x-rays and subsequently release low-energy, short-range photoelectrons during external beam radiation therapy (RT), increasing the local radiation dose. When AuNPs are near tumor vasculature, the additional radiation dose can lead to increased vascular permeability. This work focuses on understanding how tumor vascular permeability is influenced by AuNP-augmented RT, and how this effect can be used to improve the delivery of nanoparticle chemotherapeutics. Methods: Dual-energy CT was used to quantify the accumulation of both liposomal iodine and AuNPs in tumors following AuNP-augmented RT in a mouse model of primary soft tissue sarcoma. Mice were injected with non-targeted AuNPs, RGD-functionalized AuNPs (vascular targeting), or no AuNPs, after which they were treated with varying doses of RT. The mice were injected with either liposomal iodine (for the imaging study) or liposomal doxorubicin (for the treatment study) 24 hours after RT. Increased tumor liposome accumulation was assessed by dual-energy CT (iodine) or by tracking tumor treatment response (doxorubicin). Results: A significant increase in vascular permeability was observed for all groups after 20 Gy RT, for the targeted and non-targeted AuNP groups after 10 Gy RT, and for the vascular-targeted AuNP group after 5 Gy RT. Combining targeted AuNPs with 5 Gy RT and liposomal doxorubicin led to a significant tumor growth delay (tumor doubling time ~ 8 days) compared to AuNP-augmented RT or chemotherapy alone (tumor doubling time ~3-4 days). Conclusions: The addition of vascular-targeted AuNPs significantly improved the treatment effect of liposomal doxorubicin after RT, consistent with the increased liposome accumulation observed in tumors in the imaging study. Using this approach with a liposomal drug delivery system can increase specific tumor delivery of chemotherapeutics, which has the potential to significantly improve tumor response and reduce the side effects of both RT and chemotherapy.
Keywords: dual-energy CT; nanoparticles; radiation therapy; small animal imaging; tumor drug delivery; vascular imaging.
Conflict of interest statement
Competing Interests: David Kirsch is on the scientific advisory board of Lumicell Inc, which is a company commercializing intraoperative imaging and is a co-founder of XRAD Therapeutics, which is developing radiosensitizers. The other authors have declared that no competing financial interest exists.
Figures



References
-
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–13. - PubMed
-
- Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol. 2008;60:977–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

