Differences in clinical outcome between docetaxel and abiraterone acetate as the first-line treatment in chemo-naïve metastatic castration-resistant prostate cancer patients with or without the ineligible clinical factors of the COU-AA-302 study
- PMID: 29556486
- PMCID: PMC5857184
- DOI: 10.1016/j.prnil.2017.08.001
Differences in clinical outcome between docetaxel and abiraterone acetate as the first-line treatment in chemo-naïve metastatic castration-resistant prostate cancer patients with or without the ineligible clinical factors of the COU-AA-302 study
Abstract
Background: This study aimed to compare the efficacy of abiraterone acetate (AA) versus docetaxel (T) as first-line treatment in chemo-naïve metastatic castration-resistant prostate cancer (mCRPC) patients with or without the ineligible factors of the COU-AA-302 study (presence of visceral metastases, symptomatic disease, and/or Eastern Cooperative Oncology Group performance status ≥ 2).
Materials and methods: The clinical records of chemo-naïve mCRPC patients who received AA in six public oncology centers or T in two of these centers between 2003 and 2014 were reviewed. The survival time was compared among four subgroups of patients: those with ineligible factors administered AA (Group Ineligible-AA) or T (Group Ineligible-T), and those without ineligible factors and administered AA (Group Eligible-AA) or T (Group Eligible-T).
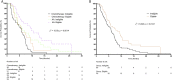
Results: During the study period, we identified 115 mCRPC patients who received AA or T, among whom 29, 36, 29, and 21 patients were classified as Groups Ineligible-AA, Ineligible-T, Eligible-AA, and Eligible-T, respectively. Both Group Ineligible-AA and Group Eligible-AA had significantly longer progression-free survival (PFS) and similar overall survival (OS) as Group Ineligible-T and Group Eligible-T (Ineligible, PFS: 6.3 vs. 5.9 months, P = 0.0234, OS: 7.8 vs. 15.7 months, P = 0.1601; Eligible, PFS: 9.8 vs. 5.6 months, P = 0.0437, OS: 20.5 vs. 18.2 months, P = 0.7820).
Conclusions: Compared to T, AA treatment resulted in longer PFS and similar OS in chemo-naïve mCRPC patients, irrespective of the presence of ineligible factors, suggesting that the initial treatment by AA may still be beneficial to those with the aforementioned ineligible factors.
Keywords: Abiraterone Acetate; Castration-Resistant Prostate Cancer; Chemo-Naïve; Chemotherapy; Metastasis.
Figures

Similar articles
-
Abiraterone acetate in metastatic castration-resistant prostate cancer - the unanticipated real-world clinical experience.BMC Urol. 2016 Mar 22;16:12. doi: 10.1186/s12894-016-0132-z. BMC Urol. 2016. PMID: 27001043 Free PMC article.
-
Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302).Eur Urol. 2014 Nov;66(5):815-25. doi: 10.1016/j.eururo.2014.02.056. Epub 2014 Mar 6. Eur Urol. 2014. PMID: 24647231 Free PMC article. Clinical Trial.
-
Subsequent Chemotherapy and Treatment Patterns After Abiraterone Acetate in Patients with Metastatic Castration-resistant Prostate Cancer: Post Hoc Analysis of COU-AA-302.Eur Urol. 2017 Apr;71(4):656-664. doi: 10.1016/j.eururo.2016.06.033. Epub 2016 Jul 9. Eur Urol. 2017. PMID: 27402060 Free PMC article.
-
Does Gleason score at initial diagnosis predict efficacy of abiraterone acetate therapy in patients with metastatic castration-resistant prostate cancer? An analysis of abiraterone acetate phase III trials.Ann Oncol. 2016 Apr;27(4):699-705. doi: 10.1093/annonc/mdv545. Epub 2015 Nov 25. Ann Oncol. 2016. PMID: 26609008 Free PMC article. Clinical Trial.
-
Abiraterone in the management of castration-resistant prostate cancer prior to chemotherapy.Ther Adv Urol. 2015 Aug;7(4):194-202. doi: 10.1177/1756287215592288. Ther Adv Urol. 2015. PMID: 26445599 Free PMC article. Review.
Cited by
-
Comparative analysis of real-world data of frequent treatment sequences in metastatic prostate cancer.Curr Urol. 2024 Jun;18(2):104-109. doi: 10.1097/CU9.0000000000000217. Epub 2024 Jun 21. Curr Urol. 2024. PMID: 39176299 Free PMC article.
-
Efficacy and safety of therapies for advanced prostate cancer in Asia: Evidence from a systematic literature review.Ther Adv Med Oncol. 2022 Nov 14;14:17588359221131525. doi: 10.1177/17588359221131525. eCollection 2022. Ther Adv Med Oncol. 2022. PMID: 36407784 Free PMC article. Review.
-
Contemporary Management of the Newly Diagnosed Prostate Cancer Patient with Metastatic Disease at Presentation.Curr Urol Rep. 2018 Aug 13;19(10):79. doi: 10.1007/s11934-018-0835-7. Curr Urol Rep. 2018. PMID: 30105573 Review.
-
Real-world first-line systemic therapy patterns in metastatic castration-resistant prostate cancer.BJUI Compass. 2021 Dec 14;3(3):205-213. doi: 10.1002/bco2.129. eCollection 2022 May. BJUI Compass. 2021. PMID: 35492221 Free PMC article.
References
-
- Schroder F., Crawford E.D., Axcrona K., Payne H., Keane T.E. Androgen deprivation therapy: past, present and future. BJU Int. 2012;109(Suppl 6):1–12. - PubMed
-
- Donkena K.V., Yuan H., Young C.Y. Recent advances in understanding hormonal therapy resistant prostate cancer. Curr Cancer Drug Targets. 2010;10:402–410. - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;(65):5–29. - PubMed
-
- Crawford E.D., Higano C.S., Shore N.D., Hussain M., Petrylak D.P. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol. 2015;194:1537–1547. - PubMed
-
- Tannock I.F., de Wit R., Berry W.R., Horti J., Pluzanska A., Chin K.N. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New Engl J Med. 2004;351:1502–1512. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
