New treatment options for inflammatory bowel diseases
- PMID: 29556726
- PMCID: PMC5910475
- DOI: 10.1007/s00535-018-1449-z
New treatment options for inflammatory bowel diseases
Abstract
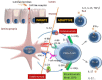
The advent of anti-TNF agents has dramatically changed the treatment algorithms for IBD in the last 15 years, but primarily and more importantly secondary loss of response is often observed. Fortunately , new treatment options have been actively explored and some have already entered our clinical practice. In the class of anti-cytokine agents, the anti-IL12/IL23 monoclonal antibodies (mAbs) have entered clinical practice with the anti-p40 mAb ustekinumab in Crohn's disease (CD). Also, more selective anti-IL23 agents (anti-p19) have shown efficacy and are being further developed, in contrast to agents inhibiting IL-17 downstream which have failed in clinical trials despite their clear efficacy in psoriasis (Verstockt et al. in Expert Opin Biol Ther 17(1):31-47, 2017; Verstockt et al. in Expert Opin Drug Saf 16(7):809-821, 2017). Following up on the efficacy of the anti-adhesion molecule vedolizumab, etrolizumab (anti-beta-7 integrin) and PF-00547659, an anti-MadCam mAb, are being developed (Lobaton et al. in Aliment Pharmacol Ther 39(6):579-594, 2014). Oral anti-trafficking agents, such as ozanimod, targeting the S1P receptor responsible for the efflux of T-cells from the lymph nodes, have also shown efficacy in patients with ulcerative colitis (UC) (Sandborn et al. in N Engl J Med 374(18):1754-1762, 2016). Oral agents inhibiting cell signaling have been explored successfully in IBD. Tofacitinib, a non-selective oral Janus kinase (JAK) inhibitor, is effective in patients with UC and several other more or less selective Jak1, 2 and 3 inhibitors are being developed for the treatment of CD and UC (Sandborn et al. in N Engl J Med 376(18):1723-1736, 2017; Vermeire et al. in Lancet 389(10066):266-275, 2017; De Vries et al. in J Crohns Colitis 11(7):885-93, 2017). Finally, despite initial disappointing results with systemic administration of mesenchymal stem cells, Alofisel, adipose tissue derived, allogeneic mesenchymal stem cells, locally injected in perianal fistula tracts, induce long-lasting beneficial effects and the drug has been approved in Europe (Panes et al. in Gastroenterology, 2017). In summary, the quest for new treatment options in IBD is very active and justified by the high medical need and unresolved problems patients are facing.
Keywords: Anti-IL23; Anti-adhesion; FMT; IBD; Jakinibs.
Conflict of interest statement
Author must indicate whether or not they have/had a financial relationship (within the last 3 years) with any organization that sponsored the research. They should also confirm that they have full control of all primary data and that they agree to allow the journal to review their data if requested.
Figures
References
-
- Verstockt B, Deleenheer B, Van Assche G, et al. A safety assessment of biological therapies targeting the IL-23/IL-17 axis in inflammatory bowel diseases. Expert Opin Drug Saf. 2017;16(7):809–821. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous