Long-term follow-up of 17 patients with childhood Pompe disease treated with enzyme replacement therapy
- PMID: 29556838
- PMCID: PMC6326992
- DOI: 10.1007/s10545-018-0166-3
Long-term follow-up of 17 patients with childhood Pompe disease treated with enzyme replacement therapy
Abstract
Objectives: Pompe disease is a progressive metabolic myopathy for which enzyme replacement therapy (ERT) was approved in 2006. While various publications have examined the effects of ERT in classic-infantile patients and in adults, little has been published on ERT in children with non-classic presentations.
Study design: This prospective study was conducted from June 1999 to May 2015. Seventeen patients from various countries participated. Outcome measures comprised muscle function (6-minute walk test, quick motor-function test (QMFT)), muscle strength (hand-held dynamometry; manual muscle testing), and lung function (FVC sitting and supine). For each outcome measure, we used linear mixed-effects models to calculate the difference at group level between the start of therapy and 7 years of ERT. Patients' individual responses over time were also evaluated.
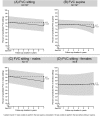
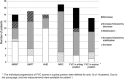
Results: Eleven males and six females started ERT at ages between 1.1 and 16.4 years (median 11.9 years); 82% of them carried the common c.-32-13T > G GAA gene variant on one allele. At group level, distance walked increased by 7.4 percentage points (p < 0.001) and QMFT scores increased by 9.2 percentage points (p = 0.006). Muscle strength scores seemed to remain stable. Results on lung function were more variable. Patients' individual data show that the proportion of patients who stabilized or improved during treatment ranged between 56 and 69% for lung function outcomes and between 71 and 93% for muscle strength and muscle function outcomes.
Conclusions: We report a positive effect of ERT in patients with childhood Pompe disease at group level. For some patients, new or personalized treatments should be considered.
Keywords: Acid maltase deficiency; Childhood; ERT; Enzyme replacement therapy; Long-term follow-up; Pompe disease.
Conflict of interest statement
Conflict of interest
Ans T. van der Ploeg has provided consultancy services to various industries in the field of Pompe disease, including Sanofi Genzyme, Biomarin and Amicus, under agreements between ErasmusMC University Medical Center and Industry. J.C. van der Meijden, M.E. Kruijshaar, L. Harlaar, D. Rizopoulos, N.A.M.E. van der Beek, declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. All patients and/or their parents provided informed consent. The medical ethical committee approved the study protocol.
Figures

References
-
- Baek RC, Palmer R, Pomponio RJ, Lu Y, Ma X, McVie-Wylie AJ. The influence of a polymorphism in the gene encoding angiotensin converting enzyme (ACE) on treatment outcomes in late-onset Pompe patients receiving alglucosidase alfa. Mol Genet Metab Rep. 2016;8:48–50. doi: 10.1016/j.ymgmr.2016.07.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

