Glioprotective Effects of Lingonberry Extract Against Altered Cellular Viability, Acetylcholinesterase Activity, and Oxidative Stress in Lipopolysaccharide-Treated Astrocytes
- PMID: 29556871
- PMCID: PMC11481962
- DOI: 10.1007/s10571-018-0581-x
Glioprotective Effects of Lingonberry Extract Against Altered Cellular Viability, Acetylcholinesterase Activity, and Oxidative Stress in Lipopolysaccharide-Treated Astrocytes
Abstract
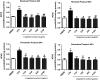
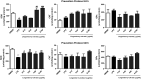
Altered astrocytic function is a contributing factor to the development of neurological diseases and neurodegeneration. Berry fruits exert neuroprotective effects by modulating pathways involved in inflammation, neurotransmission, and oxidative stress. The aim of this study was to examine the effects of the lingonberry extract on cellular viability and oxidative stress in astrocytes exposed to lipopolysaccharide (LPS). In the reversal protocol, primary astrocytic cultures were first exposed to 1 µg/mL LPS for 3 h and subsequently treated with lingonberry extract (10, 30, 50, and 100 μg/mL) for 24 and 48 h. In the prevention protocol, exposure to the lingonberry extract was performed before treatment with LPS. In both reversal and prevention protocols, the lingonberry extracts, from 10 to 100 μg/mL, attenuated LPS-induced increase in reactive oxygen species (around 55 and 45%, respectively, P < 0.01), nitrite levels (around 50 and 45%, respectively, P < 0.05), and acetylcholinesterase activity (around 45 and 60%, respectively, P < 0.05) in astrocytic cultures at 24 and 48 h. Also, in both reversal and prevention protocols, the lingonberry extract also prevented and reversed the LPS-induced decreased cellular viability (around 45 and 90%, respectively, P < 0.05), thiol content (around 55 and 70%, respectively, P < 0.05), and superoxide dismutase activity (around 50 and 145%, respectively, P < 0.05), in astrocytes at both 24 and 48 h. Our findings suggested that the lingonberry extract exerted a glioprotective effect through an anti-oxidative mechanism against LPS-induced astrocytic damage.
Keywords: Acetylcholinesterase; Astrocyte; Cellular viability; Lingonberry extract; Lipopolysaccharide; Oxidative stress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Ali SF, LeBel CP, Bondy SC (1992) Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 13(3):637–648 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

