Recent advances in medical therapy for metastatic urothelial cancer
- PMID: 29556919
- PMCID: PMC6097083
- DOI: 10.1007/s10147-018-1260-0
Recent advances in medical therapy for metastatic urothelial cancer
Abstract
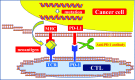
Cytotoxic chemotherapy has been the mainstay of medical therapy for metastatic urothelial cancer. Currently, the gemcitabine/cisplatin regimen is widely used worldwide as the standard first-line medical treatment. Very recently, in 2017, pembrolizumab, a highly selective, humanized monoclonal IgG4κ isotype antibody against programmed death 1, was approved as a second-line treatment to be used after platina-based chemotherapy for metastatic urothelial cancer in Japan. Based on its promising anti-tumor efficacy and manageable safety profile as demonstrated in the phase III KEYNOTE-045 trial, pembrolizumab therapy is expected to be rapidly introduced for treating metastatic urothelial cancer in clinical practice. The paradigm of medical treatment for patients with metastatic UC is dramatically changing through the introduction of this and other immune-checkpoint inhibitors. In this article, we provide a brief overview of these immune-checkpoint inhibitors and a comprehensive summary of the use of cytotoxic chemotherapy for metastatic urothelial cancer, including ongoing clinical trials.
Keywords: Chemotherapy; GC regimen; Immune-checkpoint inhibitor; MVAC regimen; Pembrolizumab; Urothelial cancer.
Conflict of interest statement
T Yuasa received remuneration for a lecture from Astellas (Tokyo, Japan), Sanofi Japan (Tokyo, Japan), Pfizer Japan (Tokyo, Japan), Novartis Pharma Japan (Tokyo, Japan), Ono Pharma (Osaka, Japan), Bristol-Myers Squibb Japan (Tokyo, Japan), and Daiichi-Sankyo (Tokyo, Japan). The other authors have declared no conflicts of interest.
Figures
References
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. - DOI - PubMed
-
- von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

