JCOG0911 INTEGRA study: a randomized screening phase II trial of interferonβ plus temozolomide in comparison with temozolomide alone for newly diagnosed glioblastoma
- PMID: 29557060
- PMCID: PMC5999164
- DOI: 10.1007/s11060-018-2831-7
JCOG0911 INTEGRA study: a randomized screening phase II trial of interferonβ plus temozolomide in comparison with temozolomide alone for newly diagnosed glioblastoma
Abstract
Purpose: This study explored the superiority of temozolomide (TMZ) + interferonβ (IFNβ) to standard TMZ as treatment for newly diagnosed glioblastoma (GBM) via randomized phase II screening design.
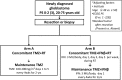
Experimental design: Eligibility criteria included histologically proven GBM, with 50% of the tumor located in supratentorial areas, without involvement of the optic, olfactory nerves, and pituitary gland and without multiple lesions and dissemination. Patients in the TMZ + radiotherapy (RT) arm received RT (2.0 Gy/fr/day, 30 fr) with TMZ (75 mg/m2, daily) followed by TMZ maintenance (100-200 mg/m2/day, days 1-5, every 4 weeks) for 2 years. Patients in the TMZ + IFNβ + RT arm intravenously received IFNβ (3 MU/body, alternative days during RT and day 1, every 4 weeks during maintenance period) and TMZ + RT. The primary endpoint was overall survival (OS). The planned sample size was 120 (one-sided alpha 0.2; power 0.8).
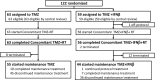
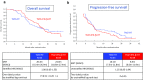
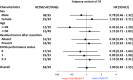
Results: Between Apr 2010 and Jan 2012, 122 patients were randomized. The median OS with TMZ + RT and TMZ + IFNβ + RT was 20.3 and 24.0 months (HR 1.00, 95% CI 0.65-1.55; one-sided log rank P = 0.51). The median progression-free survival times were 10.1 and 8.5 months (HR 1.25, 95% CI 0.85-1.84). The incidence of neutropenia with the TMZ + RT and the TMZ + IFNβ + RT (grade 3-4, CTCAE version 3.0) was 12.7 versus 20.7% during concomitant period and was 3.6 versus 9.3% during maintenance period. The incidence of lymphopenia was 54.0 versus 63.8% and 34.5 versus 41.9%.
Conclusions: TMZ + IFNβ + RT is not considered as a candidate for the following phase III trial, and TMZ + RT remained to be a most promising treatment. This trial was registered with the UMIN Clinical Trials Registry: UMIN000003466.
Keywords: Glioblastoma; Interferon-beta; MGMT; RCT; Temozolomide.
Conflict of interest statement
T.W. has received research funding from Toray Co, Ltd, and MSD Co. Ltd. K.S. received honorarium fundings from MSD. M.N. received honorarium and research fundings from MSD, Chugai, and Eisai, and research funding from Daiichi-Sankyo. R.N. received honorarium and research fundings from MSD, Chugai, and Eisai. The other authors declare that they have no conflict of interest. All authors have registered online Self-reported COI Disclosure Statement Forms through the website for Japan Neurosurgical Society members.
Figures
Similar articles
-
Genetic analysis in patients with newly diagnosed glioblastomas treated with interferon-beta plus temozolomide in comparison with temozolomide alone.J Neurooncol. 2020 May;148(1):17-27. doi: 10.1007/s11060-020-03505-9. Epub 2020 May 4. J Neurooncol. 2020. PMID: 32367437 Clinical Trial.
-
Phase I/IIa trial of fractionated radiotherapy, temozolomide, and autologous formalin-fixed tumor vaccine for newly diagnosed glioblastoma.J Neurosurg. 2014 Sep;121(3):543-53. doi: 10.3171/2014.5.JNS132392. Epub 2014 Jul 4. J Neurosurg. 2014. PMID: 24995786 Clinical Trial.
-
Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study.Neuro Oncol. 2015 May;17(5):708-17. doi: 10.1093/neuonc/nou356. Epub 2015 Mar 11. Neuro Oncol. 2015. PMID: 25762461 Free PMC article. Clinical Trial.
-
Dose Escalated Radiation Therapy for Glioblastoma Multiforme: An International Systematic Review and Meta-Analysis of 22 Prospective Trials.Int J Radiat Oncol Biol Phys. 2021 Oct 1;111(2):371-384. doi: 10.1016/j.ijrobp.2021.05.001. Epub 2021 May 12. Int J Radiat Oncol Biol Phys. 2021. PMID: 33991621
-
Hypofractionated versus standard radiation therapy in combination with temozolomide for glioblastoma in the elderly: a meta-analysis.J Neurooncol. 2019 Jun;143(2):177-185. doi: 10.1007/s11060-019-03155-6. Epub 2019 Mar 27. J Neurooncol. 2019. PMID: 30919157 Review.
Cited by
-
Combination chemoradiotherapy with temozolomide, vincristine, and interferon-β might improve outcomes regardless of O6-methyl-guanine-DNA-methyltransferase (MGMT) promoter methylation status in newly glioblastoma.BMC Cancer. 2021 Jul 28;21(1):867. doi: 10.1186/s12885-021-08592-z. BMC Cancer. 2021. PMID: 34320929 Free PMC article.
-
Current Status of Palliative and Terminal Care for Patients with Primary Malignant Brain Tumors in Japan.Neurol Med Chir (Tokyo). 2020 Dec 15;60(12):600-611. doi: 10.2176/nmc.oa.2020-0243. Epub 2020 Nov 6. Neurol Med Chir (Tokyo). 2020. PMID: 33162468 Free PMC article.
-
Analysis of transition metal content in glioblastoma reveals association between iron and survival.Transl Oncol. 2025 May;55:102376. doi: 10.1016/j.tranon.2025.102376. Epub 2025 Mar 30. Transl Oncol. 2025. PMID: 40163909 Free PMC article.
-
Dummy run study for outlining and plan quality of intensity-modulated radiotherapy in elderly patients with newly diagnosed glioblastoma: The Japan clinical oncology group study JCOG1910 (AgedGlio-PIII).Radiat Oncol. 2025 Mar 9;20(1):32. doi: 10.1186/s13014-025-02612-z. Radiat Oncol. 2025. PMID: 40059195 Free PMC article. Clinical Trial.
-
Glioblastoma and the search for non-hypothesis driven combination therapeutics in academia.Front Oncol. 2023 Jan 17;12:1075559. doi: 10.3389/fonc.2022.1075559. eCollection 2022. Front Oncol. 2023. PMID: 36733367 Free PMC article. Review.
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. - DOI - PMC - PubMed
-
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

