Neural Mechanisms of Inflammation-Induced Fever
- PMID: 29557255
- PMCID: PMC6047205
- DOI: 10.1177/1073858418760481
Neural Mechanisms of Inflammation-Induced Fever
Abstract
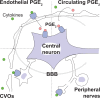
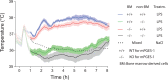
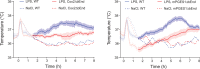
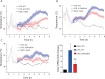
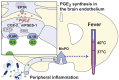
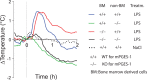
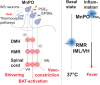
Fever is a common symptom of infectious and inflammatory disease. It is well-established that prostaglandin E2 is the final mediator of fever, which by binding to its EP3 receptor subtype in the preoptic hypothalamus initiates thermogenesis. Here, we review the different hypotheses on how the presence of peripherally released pyrogenic substances can be signaled to the brain to elicit fever. We conclude that there is unequivocal evidence for a humoral signaling pathway by which proinflammatory cytokines, through their binding to receptors on brain endothelial cells, evoke fever by eliciting prostaglandin E2 synthesis in these cells. The evidence for a role for other signaling routes for fever, such as signaling via circumventricular organs and peripheral nerves, as well as transfer into the brain of peripherally synthesized prostaglandin E2 are yet far from conclusive. We also review the efferent limb of the pyrogenic pathways. We conclude that it is well established that prostaglandin E2 binding in the preoptic hypothalamus produces fever by disinhibition of presympathetic neurons in the brain stem, but there is yet little understanding of the mechanisms by which factors such as nutritional status and ambient temperature shape the response to the peripheral immune challenge.
Keywords: EP3 receptors; brain endothelial cells; cytokines; fever; median preoptic nucleus; prostaglandin E2.
Conflict of interest statement
Figures
Similar articles
-
Immune-Induced Fever Is Dependent on Local But Not Generalized Prostaglandin E2 Synthesis in the Brain.J Neurosci. 2017 May 10;37(19):5035-5044. doi: 10.1523/JNEUROSCI.3846-16.2017. Epub 2017 Apr 24. J Neurosci. 2017. PMID: 28438967 Free PMC article.
-
Fever and hypothermia in systemic inflammation: recent discoveries and revisions.Front Biosci. 2005 Sep 1;10:2193-216. doi: 10.2741/1690. Front Biosci. 2005. PMID: 15970487 Review.
-
The afferent signalling of fever.J Physiol. 2000 Aug 1;526 Pt 3:470. J Physiol. 2000. PMID: 10921998
-
Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3.Eur J Neurosci. 2003 Oct;18(7):1848-60. doi: 10.1046/j.1460-9568.2003.02919.x. Eur J Neurosci. 2003. PMID: 14622218
-
Prostaglandin E2 production in the brainstem parabrachial nucleus facilitates the febrile response.Temperature (Austin). 2024 Sep 24;11(4):309-317. doi: 10.1080/23328940.2024.2401674. eCollection 2024. Temperature (Austin). 2024. PMID: 39583895 Free PMC article. Review.
Cited by
-
Mechanism Assay of Honeysuckle for Heat-Clearing Based on Metabolites and Metabolomics.Metabolites. 2022 Jan 27;12(2):121. doi: 10.3390/metabo12020121. Metabolites. 2022. PMID: 35208196 Free PMC article.
-
Isoorientin Inhibits Amyloid β25-35-Induced Neuronal Inflammation in BV2 Cells by Blocking the NF-κB Signaling Pathway.Molecules. 2021 Nov 22;26(22):7056. doi: 10.3390/molecules26227056. Molecules. 2021. PMID: 34834150 Free PMC article.
-
Familial Mediterranean Fever without Fever.Intern Med. 2020 May 15;59(10):1267-1270. doi: 10.2169/internalmedicine.3175-19. Epub 2020 Feb 12. Intern Med. 2020. PMID: 32051376 Free PMC article.
-
Immune Homeostasis: A Novel Example of Teamwork.Methods Mol Biol. 2024;2782:1-24. doi: 10.1007/978-1-0716-3754-8_1. Methods Mol Biol. 2024. PMID: 38622389
-
Purinergic signaling as a new mechanism underlying physical exercise benefits: a narrative review.Purinergic Signal. 2021 Dec;17(4):649-679. doi: 10.1007/s11302-021-09816-4. Epub 2021 Sep 29. Purinergic Signal. 2021. PMID: 34590239 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

