Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility
- PMID: 29558205
- PMCID: PMC6230710
- DOI: 10.1152/ajpendo.00343.2017
Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility
Abstract
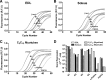
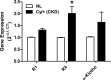
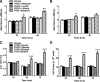
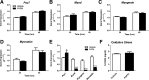
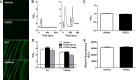
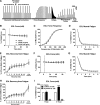
Skeletal muscle dysfunction accompanies the clinical disorders of chronic kidney disease (CKD) and hereditary hypophosphatemic rickets. In both disorders, fibroblast growth factor 23 (FGF23), a bone-derived hormone regulating phosphate and vitamin D metabolism, becomes chronically elevated. FGF23 has been shown to play a direct role in cardiac muscle dysfunction; however, it is unknown whether FGF23 signaling can also directly induce skeletal muscle dysfunction. We found expression of potential FGF23 receptors ( Fgfr1-4) and α-Klotho in muscles of two animal models (CD-1 and Cy/+ rat, a naturally occurring rat model of chronic kidney disease-mineral bone disorder) as well as C2C12 myoblasts and myotubes. C2C12 proliferation, myogenic gene expression, oxidative stress marker 8-OHdG, intracellular Ca2+ ([Ca2+]i), and ex vivo contractility of extensor digitorum longus (EDL) or soleus muscles were assessed after treatment with various amounts of FGF23. FGF23 (2-100 ng/ml) did not alter C2C12 proliferation, expression of myogenic genes, or oxidative stress after 24- to 72-h treatment. Acute or prolonged FGF23 treatment up to 6 days did not alter C2C12 [Ca2+]i handling, nor did acute treatment with FGF23 (9-100 ng/ml) affect EDL and soleus muscle contractility. In conclusion, although skeletal muscles express the receptors involved in FGF23-mediated signaling, in vitro FGF23 treatments failed to directly alter skeletal muscle development or function under the conditions tested. We hypothesize that other endogenous substances may be required to act in concert with FGF23 or apart from FGF23 to promote muscle dysfunction in hereditary hypophosphatemic rickets and CKD.
Keywords: chronic kidney disease; fibroblast growth factor 23; hypophosphatemic rickets; intracellular Ca2+; myogenesis.
Figures


References
-
- Abboud M, Rybchyn MS, Liu J, Ning Y, Gordon-Thomson C, Brennan-Speranza TC, Cole L, Greenfield H, Fraser DR, Mason RS. The effect of parathyroid hormone on the uptake and retention of 25-hydroxyvitamin D in skeletal muscle cells. J Steroid Biochem Mol Biol 173: 173–179, 2017. doi: 10.1016/j.jsbmb.2017.01.001. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

