Signal intensity analysis and optimization for in vivo imaging of Cherenkov and excited luminescence
- PMID: 29558363
- PMCID: PMC5920648
- DOI: 10.1088/1361-6560/aab83b
Signal intensity analysis and optimization for in vivo imaging of Cherenkov and excited luminescence
Abstract
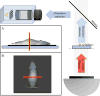
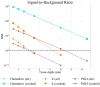
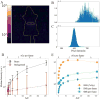
During external beam radiotherapy (EBRT), in vivo Cherenkov optical emissions can be used as a dosimetry tool or to excite luminescence, termed Cherenkov-excited luminescence (CEL) with microsecond-level time-gated cameras. The goal of this work was to develop a complete theoretical foundation for the detectable signal strength, in order to provide guidance on optimization of the limits of detection and how to optimize near real time imaging. The key parameters affecting photon production, propagation and detection were considered and experimental validation with both tissue phantoms and a murine model are shown. Both the theoretical analysis and experimental data indicate that the detection level is near a single photon-per-pixel for the detection geometry and frame rates commonly used, with the strongest factor being the signal decrease with the square of distance from tissue to camera. Experimental data demonstrates how the SNR improves with increasing integration time, but only up to the point where the dominance of camera read noise is overcome by stray photon noise that cannot be suppressed. For the current camera in a fixed geometry, the signal to background ratio limits the detection of light signals, and the observed in vivo Cherenkov emission is on the order of 100× stronger than CEL signals. As a result, imaging signals from depths <15 mm is reasonable for Cherenkov light, and depths <3 mm is reasonable for CEL imaging. The current investigation modeled Cherenkov and CEL imaging of two oxygen sensing phosphorescent compounds, but the modularity of the code allows for easy comparison of different agents or alternative cameras, geometries or tissues.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
