Coexisting genomic aberrations associated with lymph node metastasis in breast cancer
- PMID: 29558370
- PMCID: PMC5983317
- DOI: 10.1172/JCI97449
Coexisting genomic aberrations associated with lymph node metastasis in breast cancer
Abstract
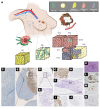
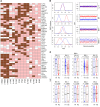
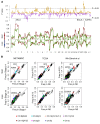
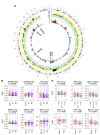
Single cancer cell-sequencing studies currently use randomly selected cells, limiting correlations among genomic aberrations, morphology, and spatial localization. We laser-captured microdissected single cells from morphologically distinct areas of primary breast cancer and corresponding lymph node metastasis and performed whole-exome or deep-target sequencing of more than 100 such cells. Two major subclones coexisted in different areas of the primary tumor, and the lymph node metastasis originated from a minor subclone in the invasive front of the primary tumor, with additional copy number changes, including chr8q gain, but no additional point mutations in driver genes. Lack of metastasis-specific driver events led us to assess whether other clonal and subclonal genomic aberrations preexisting in primary tumors contribute to lymph node metastasis. Gene mutations and copy number variations analyzed in 5 breast cancer tissue sample sets revealed that copy number variations in several genomic regions, including areas within chr1p, chr8q, chr9p, chr12q, and chr20q, harboring several metastasis-associated genes, were consistently associated with lymph node metastasis. Moreover, clonal expansion was observed in an area of morphologically normal breast epithelia, likely driven by a driver mutation and a subsequent amplification in chr1q. Our study illuminates the molecular evolution of breast cancer and genomic aberrations contributing to metastases.
Keywords: Breast cancer; Genetic variation; Genetics; Molecular genetics; Oncology.
Conflict of interest statement
Figures


Similar articles
-
Lymph Node Metastases in Colon Cancer Are Polyclonal.Clin Cancer Res. 2018 May 1;24(9):2214-2224. doi: 10.1158/1078-0432.CCR-17-1425. Epub 2017 Dec 4. Clin Cancer Res. 2018. PMID: 29203589 Free PMC article.
-
Concurrent DNA Copy-Number Alterations and Mutations in Genes Related to Maintenance of Genome Stability in Uninvolved Mammary Glandular Tissue from Breast Cancer Patients.Hum Mutat. 2015 Nov;36(11):1088-99. doi: 10.1002/humu.22845. Epub 2015 Aug 14. Hum Mutat. 2015. PMID: 26219265
-
Genetic Heterogeneity in Therapy-Naïve Synchronous Primary Breast Cancers and Their Metastases.Clin Cancer Res. 2017 Aug 1;23(15):4402-4415. doi: 10.1158/1078-0432.CCR-16-3115. Epub 2017 Mar 28. Clin Cancer Res. 2017. PMID: 28351929 Free PMC article.
-
Clonal evolution of acute myeloid leukemia highlighted by latest genome sequencing studies.Oncotarget. 2016 Sep 6;7(36):58586-58594. doi: 10.18632/oncotarget.10850. Oncotarget. 2016. PMID: 27474172 Free PMC article. Review.
-
Mechanisms of breast cancer metastasis.Clin Exp Metastasis. 2022 Feb;39(1):117-137. doi: 10.1007/s10585-021-10090-2. Epub 2021 May 5. Clin Exp Metastasis. 2022. PMID: 33950409 Free PMC article. Review.
Cited by
-
Single-cell landscape of intratumoral heterogeneity and tumor microenvironment remolding in pre-nodal metastases of breast cancer.J Transl Med. 2024 Aug 29;22(1):804. doi: 10.1186/s12967-024-05625-6. J Transl Med. 2024. PMID: 39210391 Free PMC article.
-
Genetic and microenvironmental evolution of colorectal liver metastases under chemotherapy.Cell Rep Med. 2024 Dec 17;5(12):101838. doi: 10.1016/j.xcrm.2024.101838. Epub 2024 Dec 3. Cell Rep Med. 2024. PMID: 39631402 Free PMC article.
-
Aurora Kinase A and Bcl-xL Inhibition Suppresses Metastasis in Triple-Negative Breast Cancer.Int J Mol Sci. 2022 Sep 2;23(17):10053. doi: 10.3390/ijms231710053. Int J Mol Sci. 2022. PMID: 36077449 Free PMC article.
-
MAGE-C3 promotes cancer metastasis by inducing epithelial-mesenchymal transition and immunosuppression in esophageal squamous cell carcinoma.Cancer Commun (Lond). 2021 Dec;41(12):1354-1372. doi: 10.1002/cac2.12203. Epub 2021 Aug 4. Cancer Commun (Lond). 2021. PMID: 34347390 Free PMC article.
-
Single-cell RNA sequencing reveals cell heterogeneity and transcriptome profile of breast cancer lymph node metastasis.Oncogenesis. 2021 Oct 5;10(10):66. doi: 10.1038/s41389-021-00355-6. Oncogenesis. 2021. PMID: 34611125 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

