The activity of superoxide dismutases (SODs) at the early stages of wheat deetiolation
- PMID: 29558520
- PMCID: PMC5860746
- DOI: 10.1371/journal.pone.0194678
The activity of superoxide dismutases (SODs) at the early stages of wheat deetiolation
Abstract
Unbound tetrapyrroles, i.e. protochlorophyllide (Pchlide), chlorophyllide and chlorophylls, bring the risk of reactive oxygen species (ROS) being generated in the initial stages of angiosperm deetiolation due to inefficient usage of the excitation energy for photosynthetic photochemistry. We analyzed the activity of superoxide dismutases (SODs) in etiolated wheat (Triticum aestivum) leaves and at the beginning of their deetiolation. Mn-SOD and three isoforms of Cu/Zn-SODs were identified both in etiolated and greening leaves of T. aestivum. Two Cu/Zn-SODs, denoted as II and III, were found in plastids. The activity of plastidic Cu/Zn-SOD isoforms as well as that of Mn-SOD correlated with cell aging along a monocot leaf, being the highest at leaf tips. Moreover, a high Pchlide content at leaf tips was observed. No correlation between SOD activity and the accumulation of photoactive Pchlide, i.e. Pchlide bound into ternary Pchlide:Pchlide oxidoreductase:NADPH complexes was found. Cu/Zn-SOD I showed the highest activity at the leaf base. A flash of light induced photoreduction of the photoactive Pchlide to chlorophyllide as well as an increase in all the SODs activity which occurred in a minute time-scale. In the case of seedlings that were deetiolated under continuous light of moderate intensity (100 μmol photons m-2 s-1), only some fluctuations in plastidic Cu/Zn-SODs and Mn-SOD within the first four hours of greening were noticed. The activity of SODs is discussed with respect to the assembly of tetrapyrroles within pigment-protein complexes, monitored by fluorescence spectroscopy at 77 K.
Conflict of interest statement
Figures




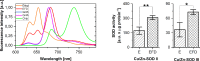
References
-
- Masuda T. Recent overview of the Mg branch of the tetrapyrrole biosynthesis leading to chlorophylls. Photosynth Res. 2008;96(2): 121–143. doi: 10.1007/s11120-008-9291-4 - DOI - PubMed
-
- Solymosi K, Schoefs B. Etioplast and etio-chloroplast formation under natural conditions: the dark side of chlorophyll biosynthesis in angiosperms. Photosynth Res. 2010;105(2): 143–166. doi: 10.1007/s11120-010-9568-2 - DOI - PubMed
-
- Brzezowski P, Richter AS, Grimm B. Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim Biophys Acta. 2015;1847(9): 968–985. doi: 10.1016/j.bbabio.2015.05.007 - DOI - PubMed
-
- Yang J, Cheng Q. Origin and evolution of the light dependent protochlorophyllide oxidoreductase (LPOR) genes. Plant Biol. 2004;6: 537–544. doi: 10.1055/s-2004-821270 - DOI - PubMed
-
- Gabruk M, Mysliwa-Kurdziel B. Light-Dependent Protochlorophyllide Oxidoreductase: Phylogeny, Regulation, and Catalytic Properties. Biochemistry. 2015;54(34): 5255–5262. doi: 10.1021/acs.biochem.5b00704 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

