Impact of RUNX2 on drug-resistant human pancreatic cancer cells with p53 mutations
- PMID: 29558908
- PMCID: PMC5861661
- DOI: 10.1186/s12885-018-4217-9
Impact of RUNX2 on drug-resistant human pancreatic cancer cells with p53 mutations
Abstract
Background: Despite the remarkable advances in the early diagnosis and treatment, overall 5-year survival rate of patients with pancreatic cancer is less than 10%. Gemcitabine (GEM), a cytidine nucleoside analogue and ribonucleotide reductase inhibitor, is a primary option for patients with advanced pancreatic cancer; however, its clinical efficacy is extremely limited. This unfavorable clinical outcome of pancreatic cancer patients is at least in part attributable to their poor response to anti-cancer drugs such as GEM. Thus, it is urgent to understand the precise molecular basis behind the drug-resistant property of pancreatic cancer and also to develop a novel strategy to overcome this deadly disease.
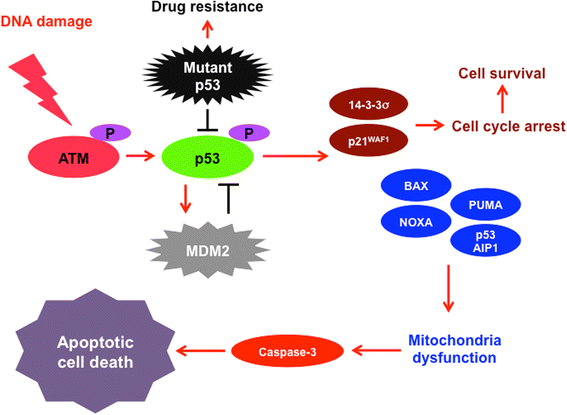
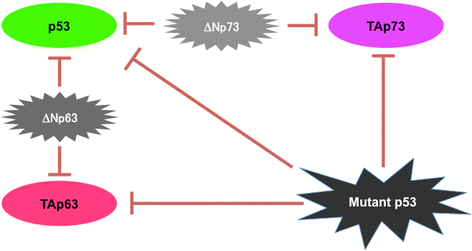
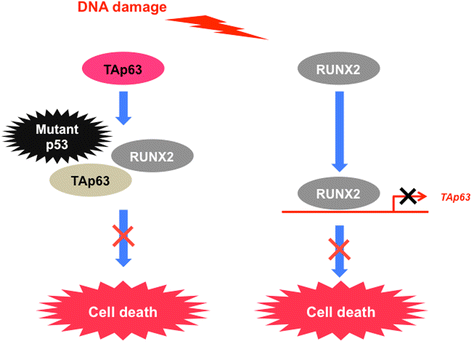
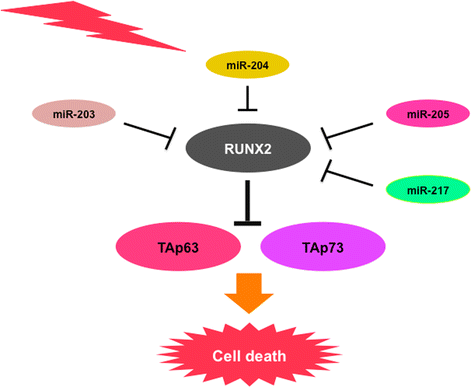
Review: Accumulating evidence strongly suggests that p53 mutations contribute to the acquisition and/or maintenance of drug-resistant property of pancreatic cancer. Indeed, certain p53 mutants render pancreatic cancer cells much more resistant to GEM, implying that p53 mutation is one of the critical determinants of GEM sensitivity. Intriguingly, runt-related transcription factor 2 (RUNX2) is expressed at higher level in numerous human cancers such as pancreatic cancer and osteosarcoma, indicating that, in addition to its pro-osteogenic role, RUNX2 has a pro-oncogenic potential. Moreover, a growing body of evidence implies that a variety of miRNAs suppress malignant phenotypes of pancreatic cancer cells including drug resistance through the down-regulation of RUNX2. Recently, we have found for the first time that forced depletion of RUNX2 significantly increases GEM sensitivity of p53-null as well as p53-mutated pancreatic cancer cells through the stimulation of p53 family TAp63/TAp73-dependent cell death pathway.
Conclusions: Together, it is likely that RUNX2 is one of the promising molecular targets for the treatment of the patients with pancreatic cancer regardless of their p53 status. In this review article, we will discuss how to overcome the serious drug-resistant phenotype of pancreatic cancer.
Keywords: Gemcitabine; Mutant p53; RUNX2; p53 family.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

