High yield production and purification of two recombinant thermostable phosphotriesterase-like lactonases from Sulfolobus acidocaldarius and Sulfolobus solfataricus useful as bioremediation tools and bioscavengers
- PMID: 29558934
- PMCID: PMC5861644
- DOI: 10.1186/s12896-018-0427-0
High yield production and purification of two recombinant thermostable phosphotriesterase-like lactonases from Sulfolobus acidocaldarius and Sulfolobus solfataricus useful as bioremediation tools and bioscavengers
Abstract
Background: Thermostable phosphotriesterase-like lactonases (PLLs) are able to degrade organophosphates and could be potentially employed as bioremediation tools and bioscavengers. But nowadays their manufacturing in high yields is still an issue that limits their industrial applications. In this work we aimed to set up a high yield production and purification biotechnological process of two recombinant PLLs expressed in E. coli, the wild type SacPox from Sulfolobus acidocaldarius and a triple mutated SsoPox C258L/I261F/W263A, originally from Sulfolobus solfataricus. To follow this aim new induction approaches were investigated to boost the enzyme production, high cell density fermentation strategies were set-up to reach higher and higher enzyme yields up to 22-L scale, a downstream train was studied to meet the requirements of an efficient industrial purification process.
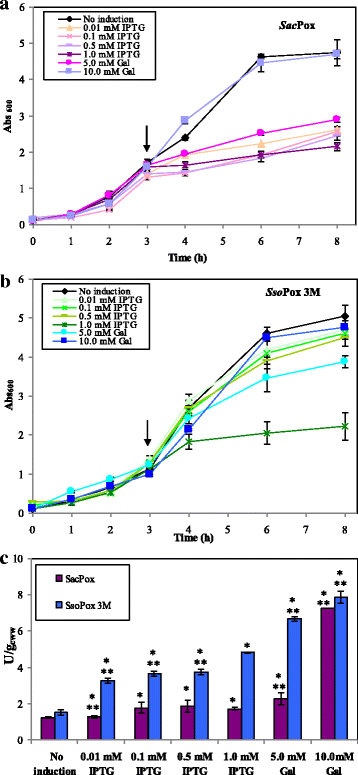
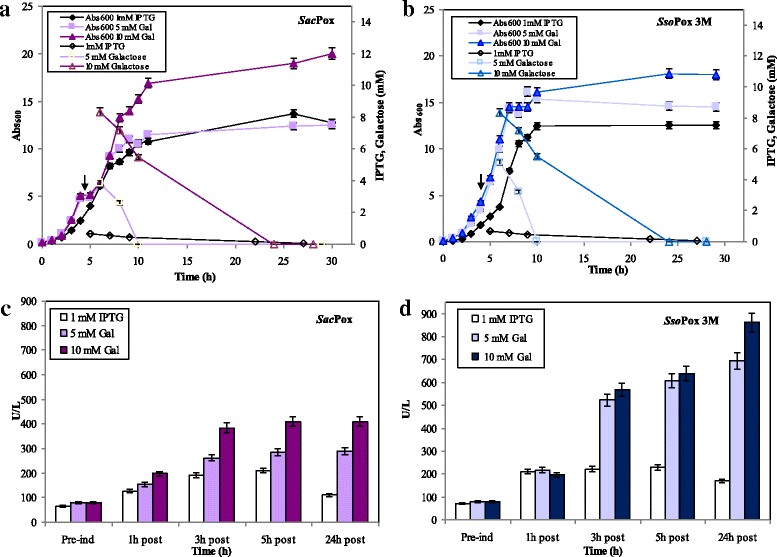
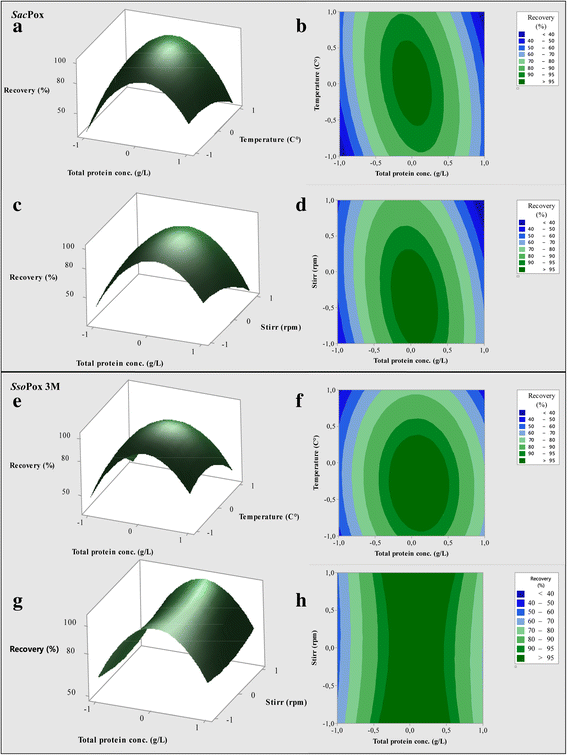
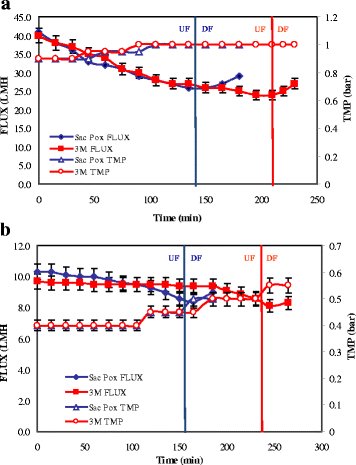
Results: Physiological studies in shake flasks demonstrated that the use of galactose as inducer increased the enzyme concentrations up to 4.5 folds, compared to the production obtained by induction with IPTG. Optimising high cell density fed-batch strategies the production and the productivity of both enzymes were further enhanced of 26 folds, up to 2300 U·L- 1 and 47.1 U·L- 1·h- 1 for SacPox and to 8700 U·L- 1 and 180.6 U·L- 1·h- 1 for SsoPox C258L/I261F/W263A, and the fermentation processes resulted scalable from 2.5 to 22.0 L. After being produced and extracted from the cells, the enzymes were first purified by a thermo-precipitation step, whose conditions were optimised by response surface methodology. A following ultra-filtration process on 100 and 5 KDa cut-off membranes drove to a final pureness and a total recovery of both enzymes of 70.0 ± 2.0%, suitable for industrial applications.
Conclusions: In this paper, for the first time, a high yield biotechnological manufacturing process of the recombinant enzymes SacPox and SsoPox C258L/I261F/W263A was set-up. The enzyme production was boosted by combining a new galactose induction approach with high cell density fed-batch fermentation strategies. An efficient enzyme purification protocol was designed coupling a thermo-precipitation step with a following membrane-based ultra-filtration process.
Keywords: Archaea; Extremozymes; Fed-batch fermentation; Organophosphates; Thermostable phosphotriesterase-like lactonase; Ultra-filtration membrane-based purification.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Jacquet P, Daudé D, Bzdrenga J, Masson P, Elias M, Chabrière E. Current and emerging strategies for organophosphate decontamination: special focus on hyperstable enzymes. Environ Sci Pollut Res. 2016; 10.1007/s11356-016-6143-1. - PubMed
-
- Ragnarsdottir KV. Environmental fate and toxicology of organophosphate pesticides. J Geol Society. 2000;157:859–876. doi: 10.1144/jgs.157.4.859. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

