Evaluation of a synthetic peptide for the detection of anti-Mycobacterium tuberculosis curli pili IgG antibodies in patients with pulmonary tuberculosis
- PMID: 29559125
- PMCID: PMC6477539
- DOI: 10.1016/j.tube.2018.01.007
Evaluation of a synthetic peptide for the detection of anti-Mycobacterium tuberculosis curli pili IgG antibodies in patients with pulmonary tuberculosis
Abstract
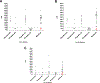
Tuberculosis (TB) remains a serious threat in underdeveloped areas. Mycobacterium tuberculosis curli pili (MTP), a virulence factor, is a potential biomarker for a reliable point of care (POC) test and was evaluated for its ability to react with Immunoglobulin G (IgG) in TB patients. An MTP synthetic peptide in a slot blot assay was used to screen serum/plasma samples (n = 65) in 3 separate cohorts, including 40 TB positive (16 HIV co-infected), 20 TB negative/HIV negative patients and 5 healthy volunteers. Forty samples were true positives (HIV positive, n = 16), 23 true negatives (HIV negative) and 2 false positives (HIV negative). The McNemar test demonstrated a 3.08% accuracy estimate (CI: -2.1% - 3.08%). This confirms that MTP is expressed during infection, including HIV-TB co-infection, is likely to be suitable for the design of a POC test and supports the validation of MTP for TB detection in larger patient populations.
Keywords: Biomarkers; Curli pili; M. tuberculosis; MTB; MTP; Tuberculosis.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


References
-
- WHO. WHO Global tuberculosis report 2016. 2016. doi:ISBN 978 92 4 156539 4.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

