ROS signaling and ER stress in cardiovascular disease
- PMID: 29559224
- PMCID: PMC6139279
- DOI: 10.1016/j.mam.2018.03.002
ROS signaling and ER stress in cardiovascular disease
Abstract
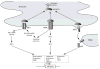
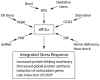
The endoplasmic reticulum (ER) produces the vast majority of all proteins secreted into the extracellular space, including hormones and cytokines, as well as cell surface receptors and other proteins which interact with the environment. Accordingly, this organelle controls essentially all vital links to a cell's external milieu, responding to systemic metabolic, inflammatory, endocrine, and mechanical stimuli. The central role the ER plays in meeting protein synthetic and quality control requirements in the face of such demands is matched by an extensive and versatile ER stress response signaling network. ROS mediate several critical aspects of this response. Nox4, an ER resident capable of producing ROS, acts as a proximal signaling intermediate to transduce ER stress-related conditions to the unfolded protein response, a homeostatic corrective mechanism. However, chronic ER stress caused by unrelenting internal or external demands produces a secondary rise in ROS, generally resulting in cell death. Sorting out the involvement of ROS at different levels of the ER stress response in specific cell types is key to understanding the molecular basis for chronic diseases such as atherosclerosis, hypertension, and diabetes. Here, we provide an overview of ER stress signaling with an emphasis on the role of ROS.
Keywords: Atherosclerosis; Autophagy; Hypertension; Nox4; Oxidative stress; Ras.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures



References
-
- Abdulkarim B, Hernangomez M, Igoillo-Esteve M, Cunha DA, Marselli L, Marchetti P, Ladriere L, Cnop M. Guanabenz Sensitizes Pancreatic beta Cells to Lipotoxic Endoplasmic Reticulum Stress and Apoptosis. Endocrinology. 2017;158(6):1659–1670. - PubMed
-
- Anilkumar N, San Jose G, Sawyer I, Santos CX, Sand C, Brewer AC, Warren D, Shah AM. A 28-kDa splice variant of NADPH oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arterioscler Thromb Vasc Biol. 2013;33(4):e104–112. - PubMed
-
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3(4):281–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

