Epidermal FABP Prevents Chemical-Induced Skin Tumorigenesis by Regulation of TPA-Induced IFN/p53/SOX2 Pathway in Keratinocytes
- PMID: 29559340
- PMCID: PMC6109432
- DOI: 10.1016/j.jid.2018.02.041
Epidermal FABP Prevents Chemical-Induced Skin Tumorigenesis by Regulation of TPA-Induced IFN/p53/SOX2 Pathway in Keratinocytes
Abstract
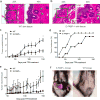
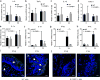
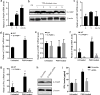
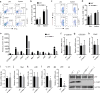
Skin lipids (e.g., fatty acids) are essential for normal skin functions. Epidermal FABP (E-FABP) is the predominant FABP expressed in skin epidermis. However, the role of E-FABP in skin homeostasis and pathology remains largely unknown. Herein, we utilized the 7,12-dimethylbenz(a)anthracene and 12-O-tetradecanolyphorbol-13-acetate-induced skin tumorigenesis model to assess the role of E-FABP in chemical-induced skin tumorigenesis. Compared to their wild-type littermates, mice deficient in E-FABP, but not adipose FABP, developed more skin tumors with higher incidence. 12-O-tetradecanolyphorbol-13-acetate functioning as a tumor promoter induced E-FABP expression and initiated extensive flaring inflammation in skin. Interestingly, 12-O-tetradecanolyphorbol-13-acetate -induced production of IFN-β and IFN-λ in the skin tissue was dependent on E-FABP expression. Further protein and gene expression arrays demonstrated that E-FABP was critical in enhancing IFN-induced p53 responses and in suppressing SOX2 expression in keratinocytes. Thus, E-FABP expression in skin suppresses chemical-induced skin tumorigenesis through regulation of IFN/p53/SOX2 pathway. Collectively, our data suggest an unknown function of E-FABP in prevention of skin tumor development, and offer E-FABP as a therapeutic target for improving skin innate immunity in chemical-induced skin tumor prevention.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors state no conflict of interest.
Figures

References
-
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le MM, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. - PubMed
-
- Crochemore C, Michaelidis TM, Fischer D, Loeffler JP, Almeida OF. Enhancement of p53 activity and inhibition of neural cell proliferation by glucocorticoid receptor activation. FASEB J. 2002;16:761–770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

