Synaptogenesis Is Modulated by Heparan Sulfate in Caenorhabditis elegans
- PMID: 29559501
- PMCID: PMC5937176
- DOI: 10.1534/genetics.118.300837
Synaptogenesis Is Modulated by Heparan Sulfate in Caenorhabditis elegans
Abstract
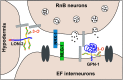
The nervous system regulates complex behaviors through a network of neurons interconnected by synapses. How specific synaptic connections are genetically determined is still unclear. Male mating is the most complex behavior in Caenorhabditis elegans It is composed of sequential steps that are governed by > 3000 chemical connections. Here, we show that heparan sulfates (HS) play a role in the formation and function of the male neural network. HS, sulfated in position 3 by the HS modification enzyme HST-3.1/HS 3-O-sulfotransferase and attached to the HS proteoglycan glypicans LON-2/glypican and GPN-1/glypican, functions cell-autonomously and nonautonomously for response to hermaphrodite contact during mating. Loss of 3-O sulfation resulted in the presynaptic accumulation of RAB-3, a molecule that localizes to synaptic vesicles, and disrupted the formation of synapses in a component of the mating circuits. We also show that the neural cell adhesion protein NRX-1/neurexin promotes and the neural cell adhesion protein NLG-1/neuroligin inhibits the formation of the same set of synapses in a parallel pathway. Thus, neural cell adhesion proteins and extracellular matrix components act together in the formation of synaptic connections.
Keywords: C. elegans; Proteoglycans; heparan sulfate; neurexin; neuroligin; synapse formation.
Copyright © 2018 Lázaro-Peña et al.
Figures





References
-
- Altun-Gultekin Z., Andachi Y., Tsalik E. L., Pilgrim D., Kohara Y., et al. , 2001. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128: 1951–1969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

