PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo
- PMID: 29559533
- PMCID: PMC5889626
- DOI: 10.1073/pnas.1713510115
PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo
Abstract
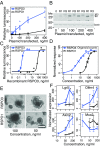
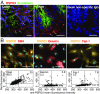
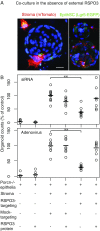
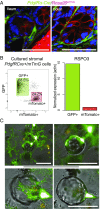
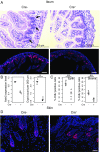
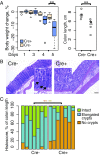
Wnts and R-spondins (RSPOs) support intestinal homeostasis by regulating crypt cell proliferation and differentiation. Ex vivo, Wnts secreted by Paneth cells in organoids can regulate the proliferation and differentiation of Lgr5-expressing intestinal stem cells. However, in vivo, Paneth cell and indeed all epithelial Wnt production is completely dispensable, and the cellular source of Wnts and RSPOs that maintain the intestinal stem-cell niche is not known. Here we investigated both the source and the functional role of stromal Wnts and RSPO3 in regulation of intestinal homeostasis. RSPO3 is highly expressed in pericryptal myofibroblasts in the lamina propria and is several orders of magnitude more potent than RSPO1 in stimulating both Wnt/β-catenin signaling and organoid growth. Stromal Rspo3 ablation ex vivo resulted in markedly decreased organoid growth that was rescued by exogenous RSPO3 protein. Pdgf receptor alpha (PdgfRα) is known to be expressed in pericryptal myofibroblasts. We therefore evaluated if PdgfRα identified the key stromal niche cells. In vivo, Porcn excision in PdgfRα+ cells blocked intestinal crypt formation, demonstrating that Wnt production in the stroma is both necessary and sufficient to support the intestinal stem-cell niche. Mice with Rspo3 excision in the PdgfRα+ cells had decreased intestinal crypt Wnt/β-catenin signaling and Paneth cell differentiation and were hypersensitive when stressed with dextran sodium sulfate. The data support a model of the intestinal stem-cell niche regulated by both Wnts and RSPO3 supplied predominantly by stromal pericryptal myofibroblasts marked by PdgfRα.
Keywords: R-spondins; Wnt signaling; intestine; myofibroblasts; stroma.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. - PubMed
-
- Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. - PubMed
-
- Storm EE, et al. Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature. 2016;529:97–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

