TgTKL1 Is a Unique Plant-Like Nuclear Kinase That Plays an Essential Role in Acute Toxoplasmosis
- PMID: 29559568
- PMCID: PMC5874906
- DOI: 10.1128/mBio.00301-18
TgTKL1 Is a Unique Plant-Like Nuclear Kinase That Plays an Essential Role in Acute Toxoplasmosis
Abstract
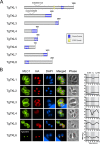
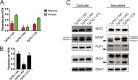
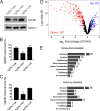
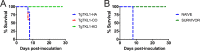
In the protozoan parasite Toxoplasma gondii, protein kinases have been shown to play key roles in regulating parasite motility, invasion, replication, egress, and survival within the host. The tyrosine kinase-like (TKL) family of proteins are an unexplored set of kinases in Toxoplasma Of the eight annotated TKLs in the Toxoplasma genome, a recent genome-wide loss-of-function screen showed that six are important for tachyzoite fitness. By utilizing an endogenous tagging approach, we showed that these six T. gondii TKLs (TgTKLs) localize to various subcellular compartments, including the nucleus, the cytosol, the inner membrane complex, and the Golgi apparatus. To gain insight into the function of TKLs in Toxoplasma, we first characterized TgTKL1, which contains the plant-like enhanced disease resistance 1 (EDR1) domain and localizes to the nucleus. TgTKL1 knockout parasites displayed significant defects in progression through the lytic cycle; we show that the defects were due to specific impairment of host cell attachment. Transcriptomics analysis identified over 200 genes of diverse functions that were differentially expressed in TgTKL1 knockout parasites. Importantly, numerous genes implicated in host cell attachment and invasion were among those most significantly downregulated, resulting in defects in microneme secretion and processing. Significantly, all of the mice inoculated intraperitoneally with TgTKL1 knockout parasites survived the infection, suggesting that TgTKL1 plays an essential role in acute toxoplasmosis. Together, these findings suggest that TgTKL1 mediates a signaling pathway that regulates the expression of multiple factors required for parasite virulence, underscoring the potential of this kinase as a novel therapeutic target.IMPORTANCEToxoplasma gondii is a protozoan parasite that can cause chronic and life-threatening disease in mammals; new drugs are greatly needed for treatment. One attractive group of drug targets consists of parasite kinases containing unique features that distinguish them from host proteins. In this report, we identify and characterize a previously unstudied kinase, TgTKL1, that localizes to the nucleus and contains a domain architecture unique to plants and protozoa. By disrupting TgTKL1, we showed that this kinase is required for the proper expression of hundreds of genes, including many that are required for the parasite to gain entry into the host cell. Specifically, parasites lacking TgTKL1 have defects in host cell attachment, resulting in impaired growth in vitro and a complete loss of virulence in mice. This report provides insight into the importance of the parasite tyrosine kinase-like kinases and establishes TgTKL1 as a novel and essential virulence factor in Toxoplasma.
Keywords: Toxoplasma gondii; apicomplexan parasites; host cell invasion; kinase.
Copyright © 2018 Varberg et al.
Figures


Similar articles
-
Kinase function of TgTKL1 is essential for its role in Toxoplasma propagation and pathogenesis.mSphere. 2024 Nov 21;9(11):e0077924. doi: 10.1128/msphere.00779-24. Epub 2024 Oct 30. mSphere. 2024. PMID: 39475314 Free PMC article.
-
TgTKL4 Is a Novel Kinase That Plays an Important Role in Toxoplasma Morphology and Fitness.mSphere. 2023 Apr 20;8(2):e0064922. doi: 10.1128/msphere.00649-22. Epub 2023 Feb 14. mSphere. 2023. PMID: 36786615 Free PMC article.
-
Characterization and functional analysis of Toxoplasma Golgi-associated proteins identified by proximity labeling.mBio. 2024 Nov 13;15(11):e0238024. doi: 10.1128/mbio.02380-24. Epub 2024 Sep 30. mBio. 2024. PMID: 39345210 Free PMC article.
-
Preparing for an invasion: charting the pathway of adhesion proteins to Toxoplasma micronemes.Parasitol Res. 2006 Apr;98(5):389-95. doi: 10.1007/s00436-005-0062-2. Epub 2005 Dec 30. Parasitol Res. 2006. PMID: 16385407 Review.
-
Modulation of innate immunity by Toxoplasma gondii virulence effectors.Nat Rev Microbiol. 2012 Nov;10(11):766-78. doi: 10.1038/nrmicro2858. Nat Rev Microbiol. 2012. PMID: 23070557 Free PMC article. Review.
Cited by
-
Temporal and thermal profiling of the Toxoplasma proteome implicates parasite Protein Phosphatase 1 in the regulation of Ca2+-responsive pathways.Elife. 2022 Aug 17;11:e80336. doi: 10.7554/eLife.80336. Elife. 2022. PMID: 35976251 Free PMC article.
-
Kinase function of TgTKL1 is essential for its role in Toxoplasma propagation and pathogenesis.mSphere. 2024 Nov 21;9(11):e0077924. doi: 10.1128/msphere.00779-24. Epub 2024 Oct 30. mSphere. 2024. PMID: 39475314 Free PMC article.
-
Prophylactic Effect of Microwave Radiation on Toxoplasma gondii Tachyzoites of RH Strain: A Method for Partial Immunization in BALB/c Mice.J Parasitol Res. 2025 May 27;2025:1666892. doi: 10.1155/japr/1666892. eCollection 2025. J Parasitol Res. 2025. PMID: 40463489 Free PMC article.
-
Protein kinases in Toxoplasma gondii.Int J Parasitol. 2021 May;51(6):415-429. doi: 10.1016/j.ijpara.2020.11.006. Epub 2021 Feb 11. Int J Parasitol. 2021. PMID: 33581139 Free PMC article. Review.
-
TgTKL4 Is a Novel Kinase That Plays an Important Role in Toxoplasma Morphology and Fitness.mSphere. 2023 Apr 20;8(2):e0064922. doi: 10.1128/msphere.00649-22. Epub 2023 Feb 14. mSphere. 2023. PMID: 36786615 Free PMC article.
References
-
- Kumar R, Adams B, Oldenburg A, Musiyenko A, Barik S. 2002. Characterisation and expression of a PP1 serine/threonine protein phosphatase (PfPP1) from the malaria parasite, Plasmodium falciparum: demonstration of its essential role using RNA interference. Malar J 1:5. doi:10.1186/1475-2875-1-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

