Interleukin-1β Signaling in Dendritic Cells Induces Antiviral Interferon Responses
- PMID: 29559569
- PMCID: PMC5874908
- DOI: 10.1128/mBio.00342-18
Interleukin-1β Signaling in Dendritic Cells Induces Antiviral Interferon Responses
Abstract
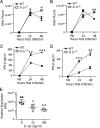
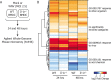
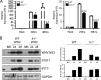
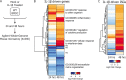
Induction of interferon beta (IFN-β), IFN-stimulated genes (ISGs), and inflammatory responses is critical for control of viral infection. We recently identified an essential linkage of stimulation of the inflammatory cytokine interleukin-1β (IL-1β) and induction of ISGs that function as host restriction pathways against the emerging flavivirus West Nile virus (WNV) in vivo Here we utilized ex vivo global transcriptome analysis of primary dendritic cells, known targets of WNV replication, to define gene signatures required for this IL-1β-driven antiviral response. Dendritic cells that were deficient in IL-1 receptor signaling showed dysregulation of cell-intrinsic defense genes and loss of viral control during WNV infection. Surprisingly, we found that in wild-type cells, IL-1β treatment, in the absence of infection, drove the transcription of IFN-β and ISGs at late times following treatment. Expression of these antiviral innate immune genes was dependent on the transcription factor IFN regulatory factor 3 (IRF3) and appears to reflect a general shift in IL-1β signaling from an early inflammatory response to a late IFN-mediated response. These data demonstrate that inflammatory and antiviral signals integrate to control viral infection in myeloid cells through a process of IL-1β-to-IRF3 signaling crosstalk. Strategies to exploit these cytokines in the activation of host defense programs should be investigated as novel therapeutic approaches against individual pathogens.IMPORTANCE West Nile virus is an emerging mosquito-borne flavivirus that can result in serious illness, neuropathology, and death in infected individuals. Currently, there are no vaccines or therapies for human use against West Nile virus. Immune control of West Nile virus infection requires inflammatory and antiviral responses, though the effect that each arm of this response has on the other is unclear. The significance of our research is in defining how virus-induced inflammatory responses regulate critical antiviral immune programs for effective control of West Nile virus infection. These data identify essential mechanisms of immune control that can inform therapeutic efforts against West Nile virus, with potential efficacy against other neuroinvasive viruses.
Keywords: IL-1; West Nile virus; flavivirus; genomics; inflammasome; innate immunity; interferon; virus.
Copyright © 2018 Aarreberg et al.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
