Chloroplast Ca2+ Fluxes into and across Thylakoids Revealed by Thylakoid-Targeted Aequorin Probes
- PMID: 29559589
- PMCID: PMC5933129
- DOI: 10.1104/pp.18.00027
Chloroplast Ca2+ Fluxes into and across Thylakoids Revealed by Thylakoid-Targeted Aequorin Probes
Abstract
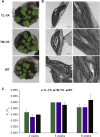
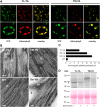
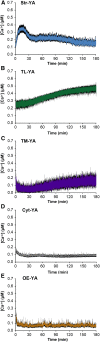
Chloroplasts require a fine-tuned control of their internal Ca2+ concentration, which is crucial for many aspects of photosynthesis and for other chloroplast-localized processes. Increasing evidence suggests that calcium regulation within chloroplasts also may influence Ca2+ signaling pathways in the cytosol. To investigate the involvement of thylakoids in Ca2+ homeostasis and in the modulation of chloroplast Ca2+ signals in vivo, we targeted the bioluminescent Ca2+ reporter aequorin as a YFP fusion to the lumen and the stromal surface of thylakoids in Arabidopsis (Arabidopsis thaliana). Thylakoid localization of aequorin-based probes in stably transformed lines was confirmed by confocal microscopy, immunogold labeling, and biochemical analyses. In resting conditions in the dark, free Ca2+ levels in the thylakoid lumen were maintained at about 0.5 μm, which was a 3- to 5-fold higher concentration than in the stroma. Monitoring of chloroplast Ca2+ dynamics in different intrachloroplast subcompartments (stroma, thylakoid membrane, and thylakoid lumen) revealed the occurrence of stimulus-specific Ca2+ signals, characterized by unique kinetic parameters. Oxidative and salt stresses initiated pronounced free Ca2+ changes in the thylakoid lumen. Localized Ca2+ increases also were observed on the thylakoid membrane surface, mirroring transient Ca2+ changes observed for the bulk stroma, but with specific Ca2+ dynamics. Moreover, evidence was obtained for dark-stimulated intrathylakoid Ca2+ changes, suggesting a new scenario for light-to-dark-induced Ca2+ fluxes inside chloroplasts. Hence, thylakoid-targeted aequorin reporters can provide new insights into chloroplast Ca2+ storage and signal transduction. These probes represent novel tools with which to investigate the role of thylakoids in Ca2+ signaling networks within chloroplasts and plant cells.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Aronsson H, Jarvis RP (2011) Rapid isolation of Arabidopsis chloroplasts and their use for in vitro protein import assays. Methods Mol Biol 774: 281–305 - PubMed
-
- Bailleul B, Berne N, Murik O, Petroutsos D, Prihoda J, Tanaka A, Villanova V, Bligny R, Flori S, Falconet D, et al. (2015) Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 524: 366–369 - PubMed
-
- Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disk assays using Evans blue. Plant Cell Tissue Organ Cult 39: 7–12
-
- Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892–895 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

