Inhibitory gain modulation of defense behaviors by zona incerta
- PMID: 29559622
- PMCID: PMC5861117
- DOI: 10.1038/s41467-018-03581-6
Inhibitory gain modulation of defense behaviors by zona incerta
Abstract
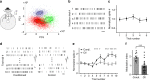
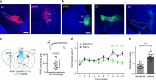
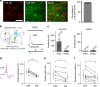
Zona incerta (ZI) is a functionally mysterious subthalamic nucleus containing mostly inhibitory neurons. Here, we discover that GABAergic neurons in the rostral sector of ZI (ZIr) directly innervate excitatory but not inhibitory neurons in the dorsolateral and ventrolateral compartments of periaqueductal gray (PAG), which can drive flight and freezing behaviors respectively. Optogenetic activation of ZIr neurons or their projections to PAG reduces both sound-induced innate flight response and conditioned freezing response, while optogenetic suppression of these neurons enhances these defensive behaviors, likely through a mechanism of gain modulation. ZIr activity progressively increases during extinction of conditioned freezing response, and suppressing ZIr activity impairs the expression of fear extinction. Furthermore, ZIr is innervated by the medial prefrontal cortex (mPFC), and silencing mPFC prevents the increase of ZIr activity during extinction and the expression of fear extinction. Together, our results suggest that ZIr is engaged in modulating defense behaviors.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Forel, A. Untersuchungen über die Haubenregion und ihre oberen Verknüpfungen im Gehirne des Menschen und einiger Säugethiere, mit Beiträgen zu den Methoden der Gehirnuntersuchung. In Archiv für Psychiatrie und Nervenkrankheiten, 7, 393–495 (1877)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

