PFKFB3 blockade inhibits hepatocellular carcinoma growth by impairing DNA repair through AKT
- PMID: 29559632
- PMCID: PMC5861039
- DOI: 10.1038/s41419-018-0435-y
PFKFB3 blockade inhibits hepatocellular carcinoma growth by impairing DNA repair through AKT
Abstract
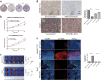
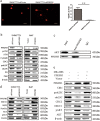
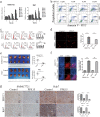
Overexpression of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), a key molecule of glucose metabolism in cytoplasm, has been found in various tumors. Emerging evidence has suggested that PFKFB3 is also located in the nucleus and apparent in regulatory functions other than glycolysis. In this study, we found that PFKFB3 expression is associated with hepatocellular carcinoma (HCC) growth and located mainly in the nucleus of tumor cells. PFKFB3 overexpression was associated with large tumor size (p = 0.04) and poor survival of patients with HCC (p = 0.027). Knockdown of PFKFB3 inhibited HCC growth, not only by reducing glucose consumption but also by damaging the DNA repair function, leading to G2/M phase arrest and apoptosis. In animal studies, overexpression of PFKFB3 is associated with increased tumor growth. Mechanistically, PFKFB3 silencing decreased AKT phosphorylation and reduced the expression of ERCC1, which is an important DNA repair protein. Moreover, PFK15, a selective PFKFB3 inhibitor, significantly inhibited tumor growth in a xenograft model of human HCC. PFKFB3 is a potential novel target in the treatment of HCC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Apatinib regulates the glycolysis of vascular endothelial cells through PI3K/AKT/PFKFB3 pathway in hepatocellular carcinoma.World J Gastroenterol. 2025 Mar 21;31(11):102848. doi: 10.3748/wjg.v31.i11.102848. World J Gastroenterol. 2025. PMID: 40124275 Free PMC article.
-
PFKFB3 is involved in breast cancer proliferation, migration, invasion and angiogenesis.Int J Oncol. 2018 Mar;52(3):945-954. doi: 10.3892/ijo.2018.4257. Epub 2018 Jan 30. Int J Oncol. 2018. PMID: 29393396
-
Inhibition of glycolytic activator PFKFB3 suppresses tumor growth and induces tumor vessel normalization in hepatocellular carcinoma.Cancer Lett. 2021 Mar 1;500:29-40. doi: 10.1016/j.canlet.2020.12.011. Epub 2020 Dec 8. Cancer Lett. 2021. PMID: 33307155
-
Nuances of PFKFB3 Signaling in Breast Cancer.Clin Breast Cancer. 2022 Jun;22(4):e604-e614. doi: 10.1016/j.clbc.2022.01.002. Epub 2022 Jan 15. Clin Breast Cancer. 2022. PMID: 35135735 Review.
-
PFKFB3 inhibitors as potential anticancer agents: Mechanisms of action, current developments, and structure-activity relationships.Eur J Med Chem. 2020 Oct 1;203:112612. doi: 10.1016/j.ejmech.2020.112612. Epub 2020 Jul 11. Eur J Med Chem. 2020. PMID: 32679452 Review.
Cited by
-
Targeting glycolytic pathway in fibroblast-like synoviocytes for rheumatoid arthritis therapy: challenges and opportunities.Inflamm Res. 2023 Dec;72(12):2155-2167. doi: 10.1007/s00011-023-01807-y. Epub 2023 Nov 8. Inflamm Res. 2023. PMID: 37940690 Review.
-
Targeting Hypoxia-Driven Metabolic Reprogramming to Constrain Tumor Progression and Metastasis.Int J Mol Sci. 2020 Jul 31;21(15):5487. doi: 10.3390/ijms21155487. Int J Mol Sci. 2020. PMID: 32751958 Free PMC article. Review.
-
The predictive value of PFKFB3 in bladder cancer prognosis.Heliyon. 2024 May 15;10(10):e31347. doi: 10.1016/j.heliyon.2024.e31347. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803949 Free PMC article.
-
Hyperglycemia induces PFKFB3 overexpression and promotes malignant phenotype of breast cancer through RAS/MAPK activation.World J Surg Oncol. 2023 Mar 28;21(1):112. doi: 10.1186/s12957-023-02990-2. World J Surg Oncol. 2023. PMID: 36973739 Free PMC article.
-
PFKFB3 Regulates Chemoresistance, Metastasis and Stemness via IAP Proteins and the NF-κB Signaling Pathway in Ovarian Cancer.Front Oncol. 2022 Jan 28;12:748403. doi: 10.3389/fonc.2022.748403. eCollection 2022. Front Oncol. 2022. PMID: 35155224 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

