Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet
- PMID: 29559675
- PMCID: PMC5861049
- DOI: 10.1038/s41598-018-23261-1
Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet
Abstract
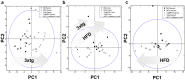
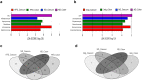
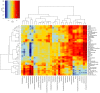
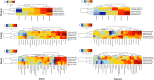
Cognitive decline, obesity and gut dysfunction or microbial dysbiosis occur in association. Our aim was to identify gut microbiota-metabolomics signatures preceding dementia in genetically prone (3xtg) mice, with and without superimposed high-fat diet. We examined the composition and diversity of their gut microbiota, and serum and faecal metabolites. 3xtg mice showed brain hypometabolism typical of pre-demented stage, and lacked the physiological bacterial diversity between caecum and colon seen in controls. Cluster analyses revealed distinct profiles of microbiota, and serum and fecal metabolome across groups. Elevation in Firmicutes-to-Bacteroidetes abundance, and exclusive presence of Turicibacteraceae, Christensenellaceae, Anaeroplasmataceae and Ruminococcaceae, and lack of Bifidobacteriaceae, were also observed. Metabolome analysis revealed a deficiency in unsaturated fatty acids and choline, and an overabundance in ketone bodies, lactate, amino acids, TMA and TMAO in 3xtg mice, with additive effects of high-fat diet. These metabolic alterations were correlated with high prevalence of Enterococcaceae, Staphylococcus, Roseburia, Coprobacillus and Dorea, and low prevalence of S24.7, rc4.4 and Bifidobacterium, which in turn related to cognitive impairment and cerebral hypometabolism. Our results indicate an effect of transgenic background on gut microbiome-metabolome, enhanced by high-fat diet. The resulting profiles may precede overt cognitive impairment, suggesting their predictive or risk-stratifying potential.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Dynamic alterations in the gut microbiota and metabolome during the development of methionine-choline-deficient diet-induced nonalcoholic steatohepatitis.World J Gastroenterol. 2018 Jun 21;24(23):2468-2481. doi: 10.3748/wjg.v24.i23.2468. World J Gastroenterol. 2018. PMID: 29930468 Free PMC article.
-
Salivary and fecal microbiota and metabolome of celiac children under gluten-free diet.Int J Food Microbiol. 2016 Dec 19;239:125-132. doi: 10.1016/j.ijfoodmicro.2016.07.025. Epub 2016 Jul 19. Int J Food Microbiol. 2016. PMID: 27452636 Review.
-
Metabolic and Gut Microbial Characterization of Obesity-Prone Mice under a High-Fat Diet.J Proteome Res. 2019 Apr 5;18(4):1703-1714. doi: 10.1021/acs.jproteome.8b00945. Epub 2019 Mar 11. J Proteome Res. 2019. PMID: 30793608
-
Comparative profiling of gut microbiota and metabolome in diet-induced obese and insulin-resistant C57BL/6J mice.Biochim Biophys Acta Mol Cell Res. 2024 Feb;1871(2):119643. doi: 10.1016/j.bbamcr.2023.119643. Epub 2023 Nov 22. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 37996062
-
METABOLIC DYSBIOSIS OF THE GUT MICROBIOTA AND ITS BIOMARKERS.Eksp Klin Gastroenterol. 2016 Jul;12(12):6-29. Eksp Klin Gastroenterol. 2016. PMID: 29889418 Review. English, Russian.
Cited by
-
Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer's Disease.Nutrients. 2020 Oct 10;12(10):3082. doi: 10.3390/nu12103082. Nutrients. 2020. PMID: 33050383 Free PMC article. Review.
-
The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease.Nutrients. 2021 Feb 25;13(3):732. doi: 10.3390/nu13030732. Nutrients. 2021. PMID: 33669008 Free PMC article.
-
The sex-specific interaction of the microbiome in neurodegenerative diseases.Brain Res. 2019 Dec 1;1724:146385. doi: 10.1016/j.brainres.2019.146385. Epub 2019 Aug 13. Brain Res. 2019. PMID: 31419428 Free PMC article. Review.
-
Prophylactic Effect of Bovine Colostrum on Intestinal Microbiota and Behavior in Wild-Type and Zonulin Transgenic Mice.Biomedicines. 2022 Dec 29;11(1):91. doi: 10.3390/biomedicines11010091. Biomedicines. 2022. PMID: 36672598 Free PMC article.
-
Predicting Neurodegenerative Disease Using Prepathology Gut Microbiota Composition: a Longitudinal Study in Mice Modeling Alzheimer's Disease Pathologies.Microbiol Spectr. 2023 Mar 6;11(2):e0345822. doi: 10.1128/spectrum.03458-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36877047 Free PMC article.
References
-
- Semar S, et al. Changes of the enteric nervous system in amyloid-β protein precursor transgenic mice correlate with disease progression. J. Alzheimer. Dis. 2013;36(1):7–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

