The theranostic efficiency of tumor-specific, pH-responsive, peptide-modified, liposome-containing paclitaxel and superparamagnetic iron oxide nanoparticles
- PMID: 29559778
- PMCID: PMC5856286
- DOI: 10.2147/IJN.S157082
The theranostic efficiency of tumor-specific, pH-responsive, peptide-modified, liposome-containing paclitaxel and superparamagnetic iron oxide nanoparticles
Abstract
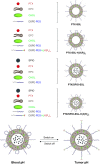
Background: In the present study, the tumor-specific, pH-responsive peptide H7K(R2)2-modified, theranostic liposome-containing paclitaxel (PTX) and superparamagnetic iron oxide nanoparticles (SPIO NPs), PTX/SPIO-SSL-H7K(R2)2, was prepared by using H7K(R2)2 as the targeting ligand, SPIO NPs as the magnetic resonance imaging (MRI) agent, PTX as antitumor drug.
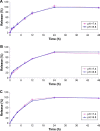
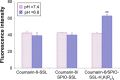
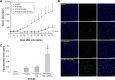
Methods: The PTX/SPIO-SSL-H7K(R2)2 was prepared by a thin film hydration method. The characteristics of PTX/SPIO-SSL-H7K(R2)2 were evaluated. The targeting effect, MRI, and antitumor activity of PTX/SPIO-SSL-H7K(R2)2 were investigated detail in vitro and in vivo in human breast carcinoma MDA-MB-231 cell models.
Results: Our results of in vitro flow cytometry, in vivo imaging, and in vivo MR imaging confirmed the pH-responsive characteristic of H7K(R2)2 in MDA-MB-231 cell line in vitro and in vivo. The results of in vivo MRI and in vivo antitumor activity confirmed the theranostic effect of PTX/SPIO-SSL-H7K(R2)2 in MDA-MB-231 tumor-bearing model.
Conclusion: Considering all our in vitro and in vivo results, we conclude that we developed targeting modified theranostic liposome which could achieve both role of antitumor and MRI.
Keywords: liposome; paclitaxel; superparamagnetic iron oxide nanoparticles; theranostic efficiency; tumor-specific pH-responsive peptide.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Ryu JH, Koo H, Sun IC, et al. Tumor-targeting multi-functional nanoparticles for theragnosis: new paradigm for cancer therapy. Adv Drug Deliv Rev. 2012;64(13):1447–1458. - PubMed
-
- Schleich N, Danhier F, Préat V. Iron oxide-loaded nanotheranostics: major obstacles to in vivo studies and clinical translation. J Control Release. 2015;198:35–54. - PubMed
-
- Hahn MA, Singh AK, Sharma P, Brown SC, Moudgil BM. Nanoparticles as contrast agents for in-vivo bioimaging: current status and future perspectives. Anal Bioanal Chem. 2011;399(1):3–27. - PubMed
-
- Bennett KM, Jo J, Cabral H, Bakalova R, Aoki I. MR imaging techniques for nano-pathophysiology and theranostics. Adv Drug Deliv Rev. 2014;74:75–94. - PubMed
-
- Roy Chowdhury M, Schumann C, Bhakta-Guha D, Guha G. Cancer nanotheranostics: strategies, promises and impediments. Biomed Pharmacother. 2016;84:291–304. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

