A cohort study on the risk of lymphoma and skin cancer in users of topical tacrolimus, pimecrolimus, and corticosteroids (Joint European Longitudinal Lymphoma and Skin Cancer Evaluation - JOELLE study)
- PMID: 29559812
- PMCID: PMC5856050
- DOI: 10.2147/CLEP.S146442
A cohort study on the risk of lymphoma and skin cancer in users of topical tacrolimus, pimecrolimus, and corticosteroids (Joint European Longitudinal Lymphoma and Skin Cancer Evaluation - JOELLE study)
Abstract
Background: There is a concern that topical tacrolimus and pimecrolimus, indicated for second-line treatment of atopic dermatitis, may increase the risk of lymphoma and skin cancer, particularly in children.
Objective: The aim of this study was to compare incidence rates (IRs) of lymphoma and skin cancer between new users of topical tacrolimus or pimecrolimus and users of moderate- to high-potency topical corticosteroids (TCSs) and untreated subjects.
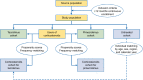
Methods: This is a multicenter cohort study with frequency matching by strata of propensity scores in population databases in the Netherlands, Denmark, Sweden, and the UK. IR ratios (IRRs) were estimated using Mantel-Haenszel methods for stratified analysis.
Results: We included 19,948 children and 66,127 adults initiating tacrolimus, 23,840 children and 37,417 adults initiating pimecrolimus, 584,121 users of TCSs, and 257,074 untreated subjects. IRs of lymphoma per 100,000 person-years were 10.4 events in children and 41.0 events in adults using tacrolimus and 3.0 events in children and 27.0 events in adults using pimecrolimus. The IRR (95% confidence interval [CI]) for lymphoma, tacrolimus versus TCSs, was 3.74 (1.00-14.06) in children and 1.27 (0.94-1.71) in adults. By lymphoma type, the highest IRR was 3.17 (0.58-17.23) for Hodgkin lymphoma in children and 1.76 (95% CI, 0.81-3.79) for cutaneous T-cell lymphoma (CTCL) in adults. For pimecrolimus versus TCSs, the highest IRR was 1.31 (95% CI, 0.33-5.14) for CTCL in adults. Compared with untreated subjects, adults using TCSs had a higher incidence of CTCL (IRR, 10.66; 95% CI, 2.60-43.75). Smaller associations were found between tacrolimus and pimecrolimus use and the risk of malignant melanoma or nonmelanoma skin cancer.
Conclusion: Use of topical tacrolimus and pimecrolimus was associated with an increased risk of lymphoma. The low IRs imply that even if the increased risk is causal, it represents a small excess risk for individual patients. Residual confounding by severity of atopic dermatitis, increased monitoring of severe patients, and reverse causation could have affected the results.
Keywords: cutaneous T-cell lymphoma; database study; malignant melanoma skin cancer; topical calcineurin inhibitors.
Conflict of interest statement
Disclosure At the time of the study, JC, LG, BC, KJR, JAK, and SP-G were full-time employees of RTI Health Solutions, an independent nonprofit research organization that does work for government agencies and pharmaceutical companies. As employees of RTI Health Solutions, SP-G, KJR, and JAK also participate in scientific advisory boards (for studies and medications) that are funded by pharmaceutical companies. JGK and MPPvH-S are employees of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for government and related health care authorities and several pharmaceutical companies. JH and AP are employees of the University of Southern Denmark, Clinical Pharmacology and Pharmacy. They have participated in studies funded by pharmaceutical companies (Alcon, Almirall, Astellas, Astra-Zeneca, Boehringer-Ingelheim, Pfizer, Menarini, Servier, and Takeda), with money paid to their employer. DD and AMG are employees of the Clinical Practice Research Datalink (CPRD), which provides contract research services for government and related health care authorities and pharmaceutical companies. IAB and AS were full-time employees of the Centre for Pharmacoepidemiology at the Karolinska Institutet during the conduct of the study. They have both taken part in studies undertaken at the Centre, financed by pharmaceutical companies, but have never received compensation personally from any company. CP is an employee of Astellas Pharma Europe. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Long-Term Risk of Skin Cancer and Lymphoma in Users of Topical Tacrolimus and Pimecrolimus: Final Results from the Extension of the Cohort Study Protopic Joint European Longitudinal Lymphoma and Skin Cancer Evaluation (JOELLE).Clin Epidemiol. 2021 Dec 29;13:1141-1153. doi: 10.2147/CLEP.S331287. eCollection 2021. Clin Epidemiol. 2021. PMID: 35002327 Free PMC article.
-
Use of Topical Tacrolimus and Topical Pimecrolimus in Four European Countries: A Multicentre Database Cohort Study.Drugs Real World Outcomes. 2018 Jun;5(2):109-116. doi: 10.1007/s40801-018-0133-1. Drugs Real World Outcomes. 2018. PMID: 29736842 Free PMC article.
-
Association between exposure to topical tacrolimus or pimecrolimus and cancers.Ann Pharmacother. 2009 Dec;43(12):1956-63. doi: 10.1345/aph.1M278. Epub 2009 Nov 10. Ann Pharmacother. 2009. PMID: 19903860
-
Safety and efficacy of topical calcineurin inhibitors in the treatment of childhood atopic dermatitis.Am J Clin Dermatol. 2005;6(2):65-77. doi: 10.2165/00128071-200506020-00001. Am J Clin Dermatol. 2005. PMID: 15799678 Review.
-
Topical pimecrolimus: a review of its clinical potential in the management of atopic dermatitis.Drugs. 2002;62(5):817-40. doi: 10.2165/00003495-200262050-00007. Drugs. 2002. PMID: 11929333 Review.
Cited by
-
Conjunctival melanoma following cornea transplant from a cancer donor: A case report.Am J Ophthalmol Case Rep. 2023 Jan 28;29:101809. doi: 10.1016/j.ajoc.2023.101809. eCollection 2023 Mar. Am J Ophthalmol Case Rep. 2023. PMID: 36793795 Free PMC article.
-
Evidence-based, Skin-directed Treatments for Cutaneous Chronic Graft-versus-host Disease.Cureus. 2019 Dec 25;11(12):e6462. doi: 10.7759/cureus.6462. Cureus. 2019. PMID: 32025391 Free PMC article. Review.
-
Topical Medications for Atopic Dermatitis and Effects on Increasing Lymphoma Risks.Cureus. 2023 Nov 20;15(11):e49135. doi: 10.7759/cureus.49135. eCollection 2023 Nov. Cureus. 2023. PMID: 38130522 Free PMC article. Review.
-
The potential for malignancy from atopic disorders and allergic inflammation: A systematic review and meta-analysis.Clin Exp Allergy. 2020 Feb;50(2):147-159. doi: 10.1111/cea.13537. Epub 2019 Dec 20. Clin Exp Allergy. 2020. PMID: 31743536 Free PMC article.
-
The Role of a Novel Generation of Emollients, 'Emollients Plus', in Atopic Dermatitis.Clin Cosmet Investig Dermatol. 2022 Dec 14;15:2705-2719. doi: 10.2147/CCID.S389697. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 36545500 Free PMC article. Review.
References
-
- European Medicines Agency Summary information on referral opinion pursuant to Article 31 of Council Directive 2001/83/EC, as amended, for Elidel and associated names [EMEA/262776/2005] Committee for Medicinal Products for Human Use (CHMP) 2006. [Accessed July 29, 2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/....
-
- European Medicines Agency [webpage on the Internet] Protopic: EPAR – Scientific Conclusion. 2006. [Accessed July 29, 2016]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici....
-
- US Food and Drug Administration Information for Healthcare Professionals: Pimecrolimus (Marketed as Elidel). FDA Alert [3/2005] 2005. [Accessed February 16, 2018]. Available from: https://wayback.archive-it.org/7993/20170406045053/https://www.fda.gov/D....
-
- US Food and Drug Administration Information for Healthcare Professionals: Tacrolimus (Marketed as Protopic). FDA Alert [3/2005] 2005. [Accessed February 16, 2018]. Available from: https://wayback.archive-it.org/7993/20170406045634/https://www.fda.gov/D....
-
- Hui RL, Lide W, Chan J, Schottinger J, Yoshinaga M, Millares M. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43(12):1956–1963. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical