Atorvastatin Attenuates Metabolic Remodeling in Ischemic Myocardium through the Downregulation of UCP2 Expression
- PMID: 29559841
- PMCID: PMC5859775
- DOI: 10.7150/ijms.22454
Atorvastatin Attenuates Metabolic Remodeling in Ischemic Myocardium through the Downregulation of UCP2 Expression
Abstract
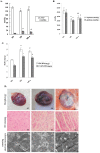
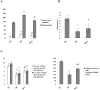
Uncoupling protein 2 (UCP2) is primarily expressed in the myocardium and is closely related to myocardial ischemia/reperfusion injury and myocardial metabolism. To explore the effects and the mechanisms of UCP2 on atorvastatin-mediated myocardium protection, the rat model of myocardial ischemia was established by ligation of the left anterior descending coronary arteries (LADs). The rats were divided into the sham operation (SO) group, myocardial infarction (MI) group and MI-atorvastatin group. The study that atorvastatin reduced myocardial remodeling and improved the disturbed myocardial energy metabolism after MI. Furthermore, the mechanisms of myocardial metabolic remodeling affected by atorvastatin were explored. The atorvastatin group showed a significantly decreased expression of UCP2 mRNA and protein. Furthermore, the primary rat cardiomyocytes were cultured and treated with angiotensin II (Ang II) to induce cardiomyocyte hypertrophy. The results showed that in the atorvastatin group, the surface area of the cardiomyocytes, the total protein content per unit of cells, and the expression of the UCP2 protein were significantly decreased. These data suggested that atorvastatin significantly attenuated the myocardial remodeling by downregulating the expression of UCP2 that was found to improve the myocardial energy metabolism, inhibit myocardial hypertrophy, and eventually reduce myocardial remodeling.
Keywords: atorvastatin; heart failure; metabolic remodeling; uncoupling proteins-2.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Tuomainen T, Tavi P. The role of cardiac energy metabolism in cardiac hypertrophy and failure. Exp Cell Res; 2017. Mar 24. pii: S0014-4827(17)30178-7. - PubMed
-
- van Bilsen M1, Smeets PJ, Gilde AJ. et al. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res. 2004;61:218–226. - PubMed
-
- Peterzan MA, Lygate CA, Neubauer S. et al. Metabolic remodelling in hypertrophied and failing myocardium: a review. Am J Physiol Heart Circ Physiol. 2017;313(3):H597–H616. - PubMed
-
- De Jong KA, Lopaschuk GD. Complex Energy Metabolic Changes in Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Can J Cardiol. 2017;33:860–871. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

