Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice
- PMID: 29559844
- PMCID: PMC5859472
- DOI: 10.7150/ijbs.23489
Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice
Abstract
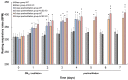
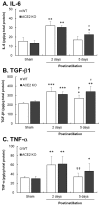
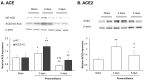
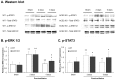
Inhaled particulate matter 2.5 (PM2.5) can cause lung injury by inducing serious inflammation in lung tissue. Renin-angiotensin system (RAS) is involved in the pathogenesis of inflammatory lung diseases and regulates inflammatory response. Angiotensin-converting enzyme II (ACE2), which is produced through the angiotensin-converting enzyme (ACE)/angiotensin II (Ang II) axis, protects against lung disease. However, few studies have focused on the relationships between PM2.5 and ACE2. Therefore, we aimed to explore the role of ACE2 in PM2.5-induced acute lung injury (ALI). An animal model of PM2.5-induced ALI was established with wild type (C57BL/6, WT) and ACE2 gene knockout (ACE2 KO) mice. The mice were exposed to PM2.5 through intratracheal instillation once a day for 3 days (6.25 mg/kg/day) and then sacrificed at 2 days and 5 days after PM2.5 instillation. The results show that resting respiratory rate (RRR), levels of inflammatory cytokines, ACE and MMPs in the lungs of WT and ACE2 KO mice were significantly increased at 2 days postinstillation. At 5 days postinstillation, the PM2.5-induced ALI significantly recovered in the WT mice, but only partially recovered in the ACE2 KO mice. The results hint that PM2.5 could induce severe ALI through pulmonary inflammation, and the repair after acute PM2.5 postinstillation could be attenuated in the absence of ACE2. Additionally, our results show that PM2.5-induced ALI is associated with signaling p-ERK1/2 and p-STAT3 pathways and ACE2 knockdown could increase pulmonary p-STAT3 and p-ERK1/2 levels in the PM2.5-induced ALI.
Keywords: acute lung injury; angiotensin-converting enzyme II; inflammation; particulate matter 2.5; renin-angiotensin system.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







References
-
- Song C, He J, Wu L. et al. Health burden attributable to ambient PM2.5 in China. Environ Pollut. 2017;223:575–586. - PubMed
-
- Churg A, Brauer M. Human lung parenchyma retains PM2.5. Am J Respir Crit Care Med. 1997;155:2109–2111. - PubMed
-
- Li YY, Lin T, Wang FW. et al. Seasonal variation of polybrominated diphenyl ethers in PM 2.5 aerosols over the East China Sea. Chemosphere. 2015;119:675–681. - PubMed
-
- Zhang Y, Ji X, Ku T. et al. Heavy metals bound to fine particulate matter from northern China induce season-dependent health risks: A study based on myocardial toxicity. Environ Pollut. 2016;216:380–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

