Characterization of MCF-12A cell phenotype, response to estrogens, and growth in 3D
- PMID: 29559854
- PMCID: PMC5859508
- DOI: 10.1186/s12935-018-0534-y
Characterization of MCF-12A cell phenotype, response to estrogens, and growth in 3D
Abstract
Background: Three-dimensional cultures of mammary epithelial cells allow for biologically-relevant studies of the development of the mammary gland in rodents and humans under normal and pathological conditions, like carcinogenesis. Under these conditions, mammotropic hormones play significant roles in tissue morphogenesis. Therefore, a system that recreates the normal, hormonally responsive epithelium would be a valuable tool to study the normal state and its transition to carcinogenesis. MCF-12A cells have been claimed to be non-tumorigenic mammary epithelial cells with reported sensitivity to estrogens. In this study, we aimed at characterizing MCF-12A cells for use in a hormone-responsive 3D culture system to determine their usefulness as a tool to identify normal and abnormal microenvironmental cues.
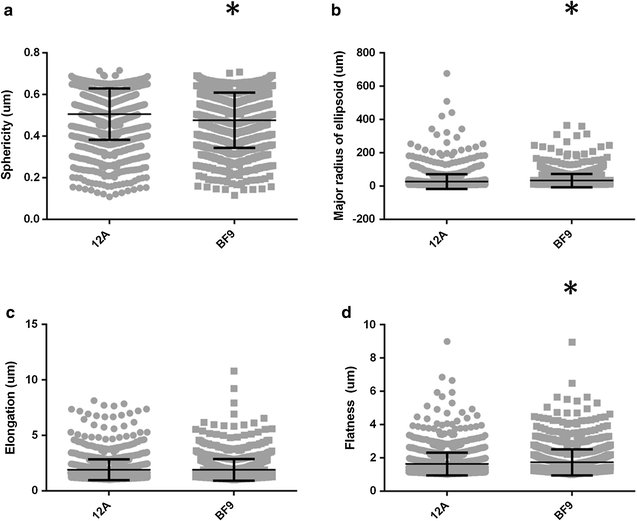
Methods: MCF-12A cells were single-cell cloned in order to investigate their heterogeneous makeup. The parental cells were then treated with estradiol to investigate proliferative and transcriptional responses through the estrogen receptor alpha. Finally, parental cells and epithelial-like cell-derived clones were seeded in rat-tail collagen I to profile the morphogenesis of multicellular 3D structures. The resultant structures were then analyzed using unsupervised morphometric analysis.
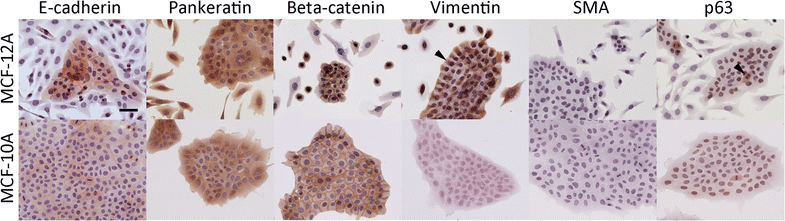
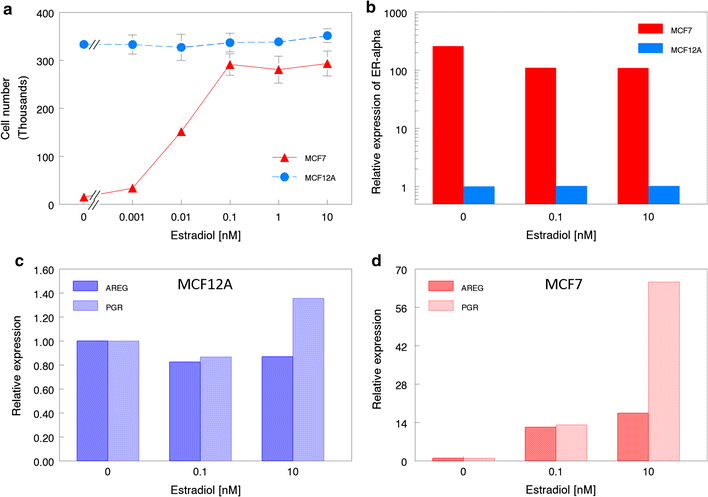
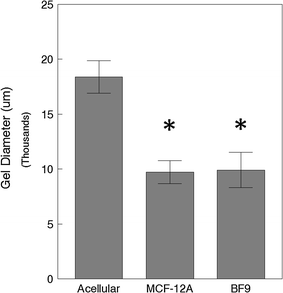
Results: MCF-12A cells consist of epithelial-like colonies which shed elongated, freely growing cells on the colony's edges. The cells express E-cadherin as well as mesenchymal vimentin but do not express markers associated with myoepithelial cells or fibroblasts. Treatment with estradiol does not affect either the proliferation rate or the induction of gene expression in MCF-12A cells. Parental MCF-12A cells form acini, solid spheres and elongated branching ducts when grown in rat-tail collagen type I matrix, the geometries and distribution of which are altered following the removal of fibroblast-like cells.
Conclusions: MCF-12A cells are a heterogeneous pseudo-epithelial cell line capable of forming a variety of multicellular structures in 3D culture. We found no indication that the cells display estrogen-responsive characteristics, thus refuting previous studies which reported estrogen responsiveness. We report that MCF-12A cells are not suited for use in studies in which differential behaviors of "normal" and "cancerous" estrogen-responsive cells are to be compared.
Keywords: 3D culture; Estrogen response; Tissue morphogenesis.
Figures

Similar articles
-
Disruption of 3D MCF-12A breast cell cultures by estrogens--an in vitro model for ER-mediated changes indicative of hormonal carcinogenesis.PLoS One. 2012;7(10):e45767. doi: 10.1371/journal.pone.0045767. Epub 2012 Oct 2. PLoS One. 2012. PMID: 23056216 Free PMC article.
-
The use of 3D cultures of MCF-10A and MCF-12A cells by high content screening for effect-based analysis of non-genotoxic carcinogens.Toxicol In Vitro. 2019 Sep;59:55-63. doi: 10.1016/j.tiv.2019.04.008. Epub 2019 Apr 8. Toxicol In Vitro. 2019. PMID: 30974152
-
A Novel Effect of β-Adrenergic Receptor on Mammary Branching Morphogenesis and its Possible Implications in Breast Cancer.J Mammary Gland Biol Neoplasia. 2017 Mar;22(1):43-57. doi: 10.1007/s10911-017-9371-1. Epub 2017 Jan 11. J Mammary Gland Biol Neoplasia. 2017. PMID: 28074314
-
ESX induces transformation and functional epithelial to mesenchymal transition in MCF-12A mammary epithelial cells.Oncogene. 2004 Mar 4;23(9):1766-79. doi: 10.1038/sj.onc.1207391. Oncogene. 2004. PMID: 14767472
-
CCAAT/Enhancer binding protein delta (c/EBPdelta) regulation and expression in human mammary epithelial cells: II. Analysis of activating signal transduction pathways, transcriptional, post-transcriptional, and post-translational control.J Cell Biochem. 2004 Nov 1;93(4):844-56. doi: 10.1002/jcb.20224. J Cell Biochem. 2004. PMID: 15389878
Cited by
-
A FACS-Free Purification Method to Study Estrogen Signaling, Organoid Formation, and Metabolic Reprogramming in Mammary Epithelial Cells.Front Endocrinol (Lausanne). 2021 Aug 12;12:672466. doi: 10.3389/fendo.2021.672466. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34456857 Free PMC article.
-
Morphometrical, Morphological, and Immunocytochemical Characterization of a Tool for Cytotoxicity Research: 3D Cultures of Breast Cell Lines Grown in Ultra-Low Attachment Plates.Toxics. 2022 Jul 24;10(8):415. doi: 10.3390/toxics10080415. Toxics. 2022. PMID: 35893848 Free PMC article.
-
Vitamin D3 constrains estrogen's effects and influences mammary epithelial organization in 3D cultures.Sci Rep. 2019 May 15;9(1):7423. doi: 10.1038/s41598-019-43308-1. Sci Rep. 2019. PMID: 31092845 Free PMC article.
-
Matrix Composition Modulates Vitamin D3's Effects on 3D Collagen Fiber Organization by MCF10A Cells.Tissue Eng Part A. 2021 Nov;27(21-22):1399-1410. doi: 10.1089/ten.TEA.2020.0371. Epub 2021 Aug 24. Tissue Eng Part A. 2021. PMID: 33789436 Free PMC article.
-
Breast cancer preclinical models: a vital resource for comprehending disease mechanisms and therapeutic development.EXCLI J. 2025 Feb 19;24:267-285. doi: 10.17179/excli2024-7973. eCollection 2025. EXCLI J. 2025. PMID: 40071025 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

