An Accumulation-of-Evidence Task Using Visual Pulses for Mice Navigating in Virtual Reality
- PMID: 29559900
- PMCID: PMC5845651
- DOI: 10.3389/fnbeh.2018.00036
An Accumulation-of-Evidence Task Using Visual Pulses for Mice Navigating in Virtual Reality
Erratum in
-
Corrigendum: An accumulation-of-evidence task using visual pulses for mice navigating in virtual reality.Front Behav Neurosci. 2022 Nov 22;16:1079746. doi: 10.3389/fnbeh.2022.1079746. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36483521 Free PMC article.
Abstract
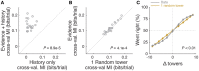
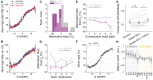
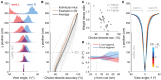
The gradual accumulation of sensory evidence is a crucial component of perceptual decision making, but its neural mechanisms are still poorly understood. Given the wide availability of genetic and optical tools for mice, they can be useful model organisms for the study of these phenomena; however, behavioral tools are largely lacking. Here, we describe a new evidence-accumulation task for head-fixed mice navigating in a virtual reality (VR) environment. As they navigate down the stem of a virtual T-maze, they see brief pulses of visual evidence on either side, and retrieve a reward on the arm with the highest number of pulses. The pulses occur randomly with Poisson statistics, yielding a diverse yet well-controlled stimulus set, making the data conducive to a variety of computational approaches. A large number of mice of different genotypes were able to learn and consistently perform the task, at levels similar to rats in analogous tasks. They are sensitive to side differences of a single pulse, and their memory of the cues is stable over time. Moreover, using non-parametric as well as modeling approaches, we show that the mice indeed accumulate evidence: they use multiple pulses of evidence from throughout the cue region of the maze to make their decision, albeit with a small overweighting of earlier cues, and their performance is affected by the magnitude but not the duration of evidence. Additionally, analysis of the mice's running patterns revealed that trajectories are fairly stereotyped yet modulated by the amount of sensory evidence, suggesting that the navigational component of this task may provide a continuous readout correlated to the underlying cognitive variables. Our task, which can be readily integrated with state-of-the-art techniques, is thus a valuable tool to study the circuit mechanisms and dynamics underlying perceptual decision making, particularly under more complex behavioral contexts.
Keywords: behavior; decision making; evidence accumulation; mouse; spatial navigation; virtual reality.
Figures





References
-
- Alyan S., Jander R. (1994). Short-range homing in the house mouse, Mus musculus: stages in the learning of directions. Anim. Behav. 48, 285–298. 10.1006/anbe.1994.1242 - DOI
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

