Molecular Mechanisms for Herpes Simplex Virus Type 1 Pathogenesis in Alzheimer's Disease
- PMID: 29559905
- PMCID: PMC5845560
- DOI: 10.3389/fnagi.2018.00048
Molecular Mechanisms for Herpes Simplex Virus Type 1 Pathogenesis in Alzheimer's Disease
Abstract
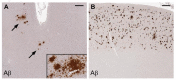
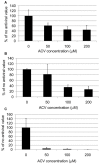
This review focuses on research in the areas of epidemiology, neuropathology, molecular biology and genetics that implicates herpes simplex virus type 1 (HSV-1) as a causative agent in the pathogenesis of sporadic Alzheimer's disease (AD). Molecular mechanisms whereby HSV-1 induces AD-related pathophysiology and pathology, including neuronal production and accumulation of amyloid beta (Aβ), hyperphosphorylation of tau proteins, dysregulation of calcium homeostasis, and impaired autophagy, are discussed. HSV-1 causes additional AD pathologies through mechanisms that promote neuroinflammation, oxidative stress, mitochondrial damage, synaptic dysfunction, and neuronal apoptosis. The AD susceptibility genes apolipoprotein E (APOE), phosphatidylinositol binding clathrin assembly protein (PICALM), complement receptor 1 (CR1) and clusterin (CLU) are involved in the HSV lifecycle. Polymorphisms in these genes may affect brain susceptibility to HSV-1 infection. APOE, for example, influences susceptibility to certain viral infections, HSV-1 viral load in the brain, and the innate immune response. The AD susceptibility gene cholesterol 25-hydroxylase (CH25H) is upregulated in the AD brain and is involved in the antiviral immune response. HSV-1 interacts with additional genes to affect cognition-related pathways and key enzymes involved in Aβ production, Aβ clearance, and hyperphosphorylation of tau proteins. Aβ itself functions as an antimicrobial peptide (AMP) against various pathogens including HSV-1. Evidence is presented supporting the hypothesis that Aβ is produced as an AMP in response to HSV-1 and other brain infections, leading to Aβ deposition and plaque formation in AD. Epidemiologic studies associating HSV-1 infection with AD and cognitive impairment are discussed. Studies are reviewed supporting subclinical chronic reactivation of latent HSV-1 in the brain as significant in the pathogenesis of AD. Finally, the rationale for and importance of clinical trials treating HSV-1-infected MCI and AD patients with antiviral medication is discussed.
Keywords: Alzheimer’s disease; amyloid beta; dementia; herpes simplex virus; neurodegeneration; neuroinflammation; pathogen; tau.
Figures





Similar articles
-
Herpes Simplex Virus Type 1 and Other Pathogens are Key Causative Factors in Sporadic Alzheimer's Disease.J Alzheimers Dis. 2015;48(2):319-53. doi: 10.3233/JAD-142853. J Alzheimers Dis. 2015. PMID: 26401998 Free PMC article. Review.
-
Alzheimer's disease plaques and tangles: cemeteries of a pyrrhic victory of the immune defence network against herpes simplex infection at the expense of complement and inflammation-mediated neuronal destruction.Neurochem Int. 2011 Feb;58(3):301-20. doi: 10.1016/j.neuint.2010.12.003. Epub 2010 Dec 15. Neurochem Int. 2011. PMID: 21167244
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Potential Involvement of Varicella Zoster Virus in Alzheimer's Disease via Reactivation of Quiescent Herpes Simplex Virus Type 1.J Alzheimers Dis. 2022;88(3):1189-1200. doi: 10.3233/JAD-220287. J Alzheimers Dis. 2022. PMID: 35754275
-
Independent and Correlated Role of Apolipoprotein E ɛ4 Genotype and Herpes Simplex Virus Type 1 in Alzheimer's Disease.J Alzheimers Dis. 2020;77(1):15-31. doi: 10.3233/JAD-200607. J Alzheimers Dis. 2020. PMID: 32804091 Review.
Cited by
-
Relation between FCGRIIB rs1050501 and HSV-1 specific IgG antibodies in Alzheimer's disease.J Transl Med. 2020 Aug 28;18(1):325. doi: 10.1186/s12967-020-02495-6. J Transl Med. 2020. PMID: 32859213 Free PMC article.
-
Tissue Microbiome Associated With Human Diseases by Whole Transcriptome Sequencing and 16S Metagenomics.Front Genet. 2021 Mar 4;12:585556. doi: 10.3389/fgene.2021.585556. eCollection 2021. Front Genet. 2021. PMID: 33747035 Free PMC article. Review.
-
Desflurane improves electrical activity of neurons and alleviates oxygen-glucose deprivation-induced neuronal injury by activating the Kcna1-dependent Kv1.1 channel.Exp Brain Res. 2024 Feb;242(2):477-490. doi: 10.1007/s00221-023-06764-w. Epub 2024 Jan 7. Exp Brain Res. 2024. PMID: 38184806
-
Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer's disease -conformist, nonconformist, and realistic prospects for AD pathogenesis.Transl Neurodegener. 2018 Dec 24;7:34. doi: 10.1186/s40035-018-0139-3. eCollection 2018. Transl Neurodegener. 2018. PMID: 30603085 Free PMC article. Review.
-
How an increase in the copy number of HSV-1 during latency can cause Alzheimer's disease: the viral and cellular dynamics according to the microcompetition model.J Neurovirol. 2021 Dec;27(6):895-916. doi: 10.1007/s13365-021-01012-9. Epub 2021 Oct 11. J Neurovirol. 2021. PMID: 34635992
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

