Allogenic Adipose Derived Stem Cells Transplantation Improved Sciatic Nerve Regeneration in Rats: Autologous Nerve Graft Model
- PMID: 29559908
- PMCID: PMC5845725
- DOI: 10.3389/fphar.2018.00086
Allogenic Adipose Derived Stem Cells Transplantation Improved Sciatic Nerve Regeneration in Rats: Autologous Nerve Graft Model
Abstract
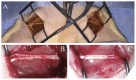
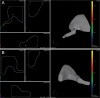
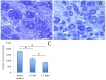
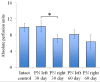
We examined the effect of transplantation of allogenic adipose-derived stem cells (ADSCs) with properties of mesenchymal stem cells (MSCs) on posttraumatic sciatic nerve regeneration in rats. We suggested an approach to rat sciatic nerve reconstruction using the nerve from the other leg as a graft. The comparison was that of a critical 10 mm nerve defect repaired by means of autologous nerve grafting versus an identical lesion on the contralateral side. In this experimental model, the same animal acts simultaneously as a test model, and control. Regeneration of the left nerve was enhanced by the use of ADSCs, whereas the right nerve healed under natural conditions. Thus the effects of individual differences were excluded and a result closer to clinical practice obtained. We observed significant destructive changes in the sciatic nerve tissue after surgery which resulted in the formation of combined contractures in knee and ankle joints of both limbs and neurotrophic ulcers only on the right limb. The stimulation of regeneration by ADSCs increased the survival of spinal L5 ganglia neurons by 26.4%, improved sciatic nerve vascularization by 35.68% and increased the number of myelin fibers in the distal nerve by 41.87%. Moreover, we have demonstrated that S100, PMP2, and PMP22 gene expression levels are suppressed in response to trauma as compared to intact animals. We have shown that ADSC-based therapy contributes to significant improvement in the regeneration.
Keywords: DRG; IVIS Spectrum; PCR; PNI; autologous nerve graft; myelin fibers.
Figures



Similar articles
-
[Effects of adipose-derived mesenchymal stem cells over-expressing glial cell line-derived neurotrophic factor on electrically injured sciatic nerve of rats].Zhonghua Shao Shang Za Zhi. 2015 Jun;31(3):199-204. Zhonghua Shao Shang Za Zhi. 2015. PMID: 26564567 Chinese.
-
Bone marrow-derived mesenchymal stem cells versus adipose-derived mesenchymal stem cells for peripheral nerve regeneration.Neural Regen Res. 2018 Jan;13(1):100-104. doi: 10.4103/1673-5374.224378. Neural Regen Res. 2018. PMID: 29451213 Free PMC article.
-
GDNF-ADSCs-APG embedding enhances sciatic nerve regeneration after electrical injury in a rat model.J Cell Biochem. 2019 Sep;120(9):14971-14985. doi: 10.1002/jcb.28759. Epub 2019 May 6. J Cell Biochem. 2019. PMID: 31062403
-
Effects of adipose derived stem cells pretreated with resveratrol on sciatic nerve regeneration in rats.Sci Rep. 2023 Apr 10;13(1):5812. doi: 10.1038/s41598-023-32906-9. Sci Rep. 2023. PMID: 37037844 Free PMC article.
-
Current progress in use of adipose derived stem cells in peripheral nerve regeneration.World J Stem Cells. 2015 Jan 26;7(1):51-64. doi: 10.4252/wjsc.v7.i1.51. World J Stem Cells. 2015. PMID: 25621105 Free PMC article. Review.
Cited by
-
Augmenting Peripheral Nerve Regeneration with Adipose-Derived Stem Cells.Stem Cell Rev Rep. 2022 Feb;18(2):544-558. doi: 10.1007/s12015-021-10236-5. Epub 2021 Aug 20. Stem Cell Rev Rep. 2022. PMID: 34417730 Free PMC article. Review.
-
Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update.Neural Regen Res. 2023 Jun;18(6):1203-1212. doi: 10.4103/1673-5374.355981. Neural Regen Res. 2023. PMID: 36453395 Free PMC article. Review.
-
A Fluorescence Resonance Energy Transfer Aptasensor for Aflatoxin B1 Based on Ligand-Induced ssDNA Displacement.Molecules. 2023 Dec 1;28(23):7889. doi: 10.3390/molecules28237889. Molecules. 2023. PMID: 38067619 Free PMC article.
-
TrkA regulates the regenerative capacity of bone marrow stromal stem cells in nerve grafts.Neural Regen Res. 2019 Oct;14(10):1765-1771. doi: 10.4103/1673-5374.257540. Neural Regen Res. 2019. PMID: 31169194 Free PMC article.
-
Adipose-Derived Mesenchymal Stem Cells Applied in Fibrin Glue Stimulate Peripheral Nerve Regeneration.Front Med (Lausanne). 2019 Apr 9;6:68. doi: 10.3389/fmed.2019.00068. eCollection 2019. Front Med (Lausanne). 2019. PMID: 31024916 Free PMC article.
References
-
- Akbulut H., Cüce G., Aktan T. M., Duman S. (2012). Expression of mesenchymal stem cell markers of human adipose tissue surrounding the vas deferens. Biomed. Res. 23 166–169.
LinkOut - more resources
Full Text Sources
Other Literature Sources

