Novel Approaches to Exploiting Invariant NKT Cells in Cancer Immunotherapy
- PMID: 29559971
- PMCID: PMC5845557
- DOI: 10.3389/fimmu.2018.00384
Novel Approaches to Exploiting Invariant NKT Cells in Cancer Immunotherapy
Abstract
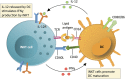
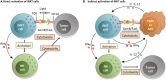
iNKT cells are a subset of innate-like T cells that utilize an invariant TCR alpha chain complexed with a limited repertoire of TCR beta chains to recognize specific lipid antigens presented by CD1d molecules. Because iNKT cells have an invariant TCR, they can be easily identified and targeted in both humans and mice via standard reagents, making this a population of T cells that has been well characterized. iNKT cells are some of the first cells to respond during an infection. By making different types of cytokines in response to different infection stimuli, iNKT cells help determine what kind of immune response then develops. It has been shown that iNKT cells are some of the first cells to respond during infection with a pathogen and the type of cytokines that iNKT cells make help determine the type of immune response that develops in various situations. Indeed, along with immunity to pathogens, pre-clinical mouse studies have clearly demonstrated that iNKT cells play a critical role in tumor immunosurveillance. They can mediate anti-tumor immunity by direct recognition of tumor cells that express CD1d, and/or via targeting CD1d found on cells within the tumor microenvironment. Multiple groups are now working on manipulating iNKT cells for clinical benefit within the context of cancer and have demonstrated that targeting iNKT cells can have a therapeutic benefit in patients. In this review, we briefly introduce iNKT cells, then discuss preclinical data on roles of iNKT cells and clinical trials that have targeted iNKT cells in cancer patients. We finally discuss how future trials could be modified to further increase the efficacy of iNKT cell therapies, in particular CAR-iNKT and rTCR-iNKT cells.
Keywords: CD1d; NKT cells; cancer immunotherapy; iNKT cells; monoclonal antibody.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

