The natural catalytic function of Cu GE glucuronoyl esterase in hydrolysis of genuine lignin-carbohydrate complexes from birch
- PMID: 29560026
- PMCID: PMC5858132
- DOI: 10.1186/s13068-018-1075-2
The natural catalytic function of Cu GE glucuronoyl esterase in hydrolysis of genuine lignin-carbohydrate complexes from birch
Abstract
Background: Glucuronoyl esterases belong to carbohydrate esterase family 15 and catalyze de-esterification. Their natural function is presumed to be cleavage of ester linkages in lignin-carbohydrate complexes particularly those linking lignin and glucuronoyl residues in xylans in hardwood.
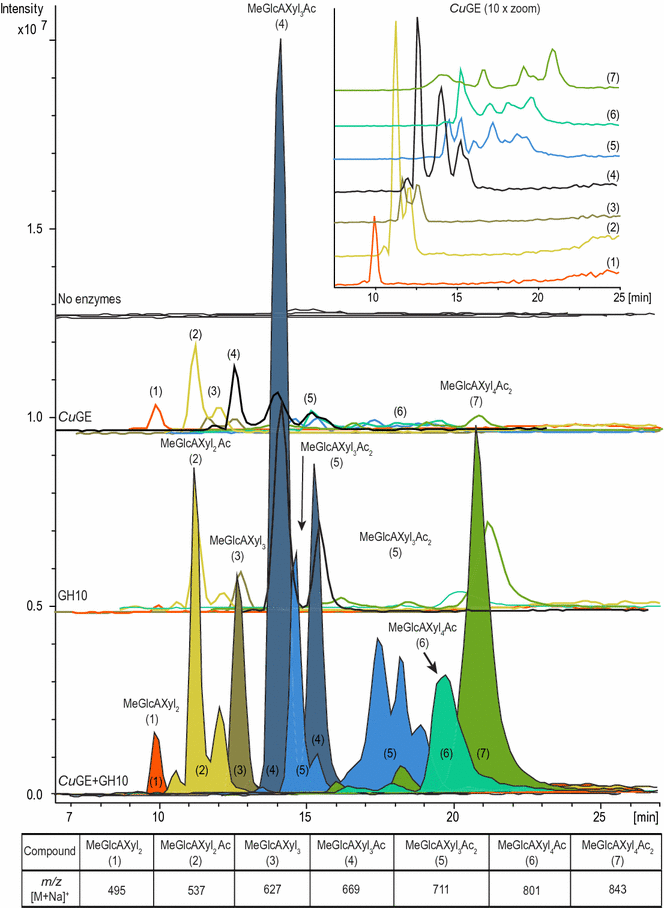
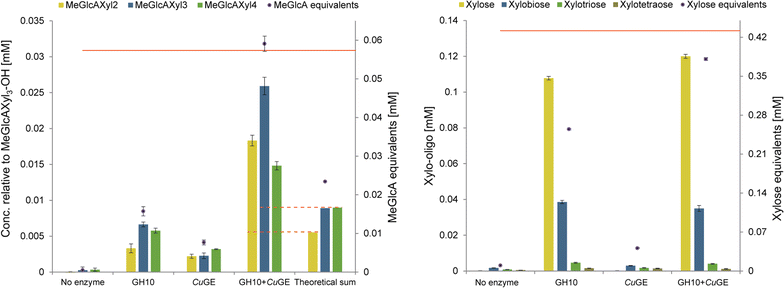
Results: Here, we show for the first time a detailed product profile of aldouronic acids released from birchwood lignin by a glucuronoyl esterase from the white-rot fungus Cerrena unicolor (CuGE). CuGE releases substrate for GH10 endo-xylanase which results in significantly increased product release compared to the action of endo-xylanase alone. CuGE also releases neutral xylo-oligosaccharides that can be ascribed to the enzymes feruloyl esterase side activity as demonstrated by release of ferulic acid from insoluble wheat arabinoxylan.
Conclusion: The data verify the enzyme's unique ability to catalyze removal of all glucuronoxylan associated with lignin and we propose that this is a direct result of enzymatic cleavage of the ester bonds connecting glucuronoxylan to lignin via 4-O-methyl glucuronoyl-ester linkages. This function appears important for the fungal organism's ability to effectively utilize all available carbohydrates in lignocellulosic substrates. In bioprocess perspectives, this enzyme is a clear candidate for polishing lignin for residual carbohydrates to achieve pure, native lignin fractions after minimal pretreatment.
Keywords: Aldouronic acids; CE15; Glucuronoxylan; Glucuronoyl esterases; LCC; Lignin.
Figures

References
-
- Takahashi N, Koshijima T. Ester linkages between lignin and glucuronoxylan in a lignin-carbohydrate complex from beech (Fagus crenata) wood. Wood Sci Technol. 1988;22:231–241. doi: 10.1007/BF00386018. - DOI
-
- Watanabe T, Koshijima T. Evidence for an ester linkage between lignin and glucuronic acid in lignin-carbohydrate complexes by DDQ-oxidation. Agric Biol Chem. 1988;52:2953–2955.
LinkOut - more resources
Full Text Sources
Other Literature Sources

