Ang-2 promotes lung cancer metastasis by increasing epithelial-mesenchymal transition
- PMID: 29560103
- PMCID: PMC5849167
- DOI: 10.18632/oncotarget.24061
Ang-2 promotes lung cancer metastasis by increasing epithelial-mesenchymal transition
Abstract
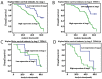
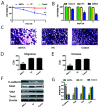
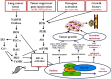
Lung cancer is the most common malignant tumor with increasing angiopoietin-2 (Ang-2) and a high rate of metastasis. However, the mechanism of Ang-2 enhancing tumor proliferation and facilitating metastasis remains to be clarified. In this study, Ang-2 expression and its gene transcription on effects of biological behaviors and epithelial-mesenchymal transition (EMT) were investigated in lung cancers. Total incidence of Ang-2 expression in the cancerous tissues was up to 91.8 % (112 of 122) with significantly higher (χ2=103.753, P2=7.883, P=0.005), differentiation degree (χ2=4.554, P=0.033), tumor node metastasis (TNM) staging (χ2=5.039, P=0.025), and 5-year survival rate (χ2 =11.220, P2=18.881, P2=0.81, P=0.776) or III & IV (χ2=1.845, P=0.174). Over-expression of Ang-2 or Ang-2 mRNA in lung A549 and NCI-H1975 cells were identified among different cell lines. When silencing Ang-2 in A549 cells with specific shRNA-1 transfection, the cell proliferation was significantly inhibited in a time-dependent manner, with up-regulating E-cadherin, down-regulating Vimentin, Twist, and Snail expression, and decreasing invasion and metastasis of cancer cell abilities, suggesting that Ang-2 promote tumor metastasis through increasing EMT, and it could be a potential target for lung cancer therapy.
Keywords: Ang-2; EMT; RNA interference; lung cancer; prognosis.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest were disclosed.
Figures


References
-
- Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann Oncol. 2017;28:1117–1123. - PubMed
-
- Cassidy RJ, Zhang X, Patel PR, Shelton JW, Escott CE, Sica GL, Rossi MR, Hill CE, Steuer CE, Pillai RN, Ramalingam SS, Owonikoko TK, Behera M, et al. Next-generation sequencing and clinical outcomes of patients with lung adenocarcinoma treated with stereotactic body radiotherapy. Cancer. 2017;123:3681–3690. - PubMed
-
- Brock M, Mei Y. Protein functional effector sncRNAs (pfeRNAs) in lung cancer. Cancer Lett. 2017;403:138–143. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

