Large, heterometallic coordination cages based on ditopic metallo-ligands with 3-pyridyl donor groups
- PMID: 29560187
- PMCID: PMC5811078
- DOI: 10.1039/c4sc03046j
Large, heterometallic coordination cages based on ditopic metallo-ligands with 3-pyridyl donor groups
Abstract
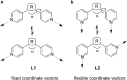
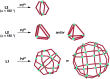
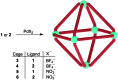
Ditopic N-donor ligands with terminal 4-pyridyl groups are omnipresent in coordination-based self-assembly. The utilization of ligands with 3-pyridyl donor groups is significantly less common, because the intrinsic conformational flexibility of these ligands tends to favor the formation of small aggregates. Here, we show that large Pd6L1212+ cages can be obtained by reaction of Pd(ii) salts with metallo-ligands L bearing terminal 3-pyridyl groups. The easy-to-access metallo-ligands contain an Fe(ii) clathrochelate core. These sterically demanding clathrochelate complexes prevent the formation of smaller aggregates, which is observed for less bulky analogous building blocks. The cages were shown to bind BF4- and BPh4- anions in aqueous solvent mixtures, whilst the lateral size of the clathrochelate significantly affects their guest encapsulation behavior.
Figures


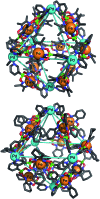
References
-
-
For selected recent review articles about molecularly defined assemblies see:
- Mukherjee S., Mukherjee P. S. Chem. Commun. 2014;50:2239. - PubMed
- Ward M. D., Raithby P. R. Chem. Soc. Rev. 2013;42:1619. - PubMed
- Hardy J. G. Chem. Soc. Rev. 2013;42:7881. - PubMed
- Young N. J., Hay B. P. Chem. Commun. 2013;49:1354. - PubMed
- Ronson T. K., Zarra S., Black S. P., Nitschke J. R. Chem. Commun. 2013;49:2476. - PubMed
- Amouri H., Desmarets C., Moussa J. Chem. Rev. 2012;112:2015. - PubMed
- Chakrabarty R., Mukherjee P. S., Stang P. J. Chem. Rev. 2011;111:6810. - PMC - PubMed
- Wiester M. J., Ulmann P. A., Mirkin C. A. Angew. Chem., Int. Ed. 2011;50:114. - PubMed
-
-
-
For selected recent review articles about polymeric assemblies see:
- Lu W., Wei Z., Gu Z.-Y., Liu T.-F., Park J., Park J., Tina J., Zhang M., Zhang Q., Gentle III T., Bosch M., Zhou H.-C. Chem. Soc. Rev. 2014;43:5561–5593. - PubMed
- Guillerm V., Kim D., Eubank J. F., Luebke R., Liu X., Adil K., Lah M. S., Eddaoudi M. Chem. Soc. Rev. 2014;43:6141. - PubMed
- Furukawa H., Cordova K. E., O'Keeffe M., Yaghi O. M. Science. 2013;341:974. - PubMed
- Cook T. R., Zheng Y.-R., Stang P. J. Chem. Rev. 2013;113:734. - PMC - PubMed
- Xuan W., Zhu C., Liu Y., Cui Y. Chem. Soc. Rev. 2012;41:1677. - PubMed
- Adarsh N. N., Dastidar P. Chem. Soc. Rev. 2012;41:3039. - PubMed
- Almeida F. A., Klinowski J., Vilela S. M. F., Tomé J. P. C., Cavaleiro J. A. S., Rocha J. Chem. Soc. Rev. 2012;41:1088. - PubMed
- Stock N., Biswas S. Chem. Rev. 2012;112:933. - PubMed
-
-
- Review: Harris K., Fujita D., Fujita M., Chem. Commun., 2013, 49 , 6703 . - PubMed
-
-
Selected examples:
- Huang S.-L., Lin Y.-J., Hor T. S. A., Jin G.-X. J. Am. Chem. Soc. 2013;135:8125. - PubMed
- Fujita D., Suzuki K., Sato S., Yagi-Utsumi M., Yamaguchi Y., Mizuno N., Kumasaka T., Takata M., Noda M., Uchiyama S., Kato K., Fujita M. Nat. Commun. 2012;3:1093. - PubMed
- Bunsen J., Iwasa J., Bonakdarzadeh P., Numata E., Rissanen K., Sato S., Fujita M. Angew. Chem., Int. Ed. 2012;51:3161. - PubMed
- Sun Q.-F., Murase T., Sato S., Fujita M. Angew. Chem., Int. Ed. 2011;50:10318. - PubMed
- Sun Q.-F., Iwasa J., Ogawa D., Ishido Y., Sato S., Ozeki T., Sei Y., Yamaguchi K., Fujita M. Science. 2010;328:1144. - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

