Tyrosine-derived stimuli responsive, fluorescent amino acids
- PMID: 29560202
- PMCID: PMC5811119
- DOI: 10.1039/c4sc02753a
Tyrosine-derived stimuli responsive, fluorescent amino acids
Abstract
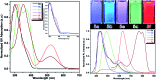
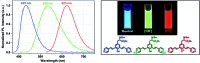
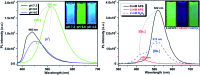
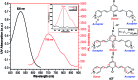
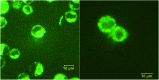
A series of fluorescent unnatural amino acids (UAAs) bearing stilbene and meta-phenylenevinylene (m-PPV) backbone have been synthesized and their optical properties were studied. These novel UAAs were derived from protected diiodo-l-tyrosine using palladium-catalyzed Heck couplings with a series of styrene analogs. Unlike the other fluorescent UAAs, whose emissions are restricted to a narrow range of wavelengths, these new amino acids display the emission peaks at broad range wavelengths (from 400-800 nm); including NIR with QY of 4% in HEPES buffer. The incorporation of both pyridine and phenol functional groups leads to distinct red, green, and blue (RGB) emission, in its basic, acidic and neutral states, respectively. More importantly, these amino acids showed reversible pH and redox response showing their promise as stimuli responsive fluorescent probes. To further demonstrate the utility of these UAAs in peptide synthesis, one of the amino acids was incorporated into a cell penetrating peptide (CPP) sequence through standard solid phase peptide synthesis. Resultant CPP was treated with two different cell lines and the internalization was monitored by confocal fluorescence microscopy.
Figures




Similar articles
-
Generating permissive site-specific unnatural aminoacyl-tRNA synthetases.Biochemistry. 2010 Mar 2;49(8):1667-77. doi: 10.1021/bi901947r. Biochemistry. 2010. PMID: 20082521
-
Synthesis and Protein Incorporation of Azido-Modified Unnatural Amino Acids.RSC Adv. 2015;5(2):1274-1281. doi: 10.1039/C4RA14244F. Epub 2014 Dec 2. RSC Adv. 2015. PMID: 26478813 Free PMC article.
-
Cell-Penetrating Peptides Containing Fluorescent d-Cysteines.Chemistry. 2018 Jun 4;24(31):7991-8000. doi: 10.1002/chem.201800603. Epub 2018 May 8. Chemistry. 2018. PMID: 29603441
-
Unnatural amino acids: production and biotechnological potential.World J Microbiol Biotechnol. 2019 Apr 8;35(4):67. doi: 10.1007/s11274-019-2642-9. World J Microbiol Biotechnol. 2019. PMID: 30963257 Review.
-
The Benefits of Unnatural Amino Acid Incorporation as Protein Labels for Single Molecule Localization Microscopy.Front Chem. 2021 Mar 25;9:641355. doi: 10.3389/fchem.2021.641355. eCollection 2021. Front Chem. 2021. PMID: 33842432 Free PMC article. Review.
Cited by
-
Synthesis of Fluorescent Dibenzofuran α-Amino Acids: Conformationally Rigid Analogues of Tyrosine.Org Lett. 2025 Mar 14;27(10):2475-2479. doi: 10.1021/acs.orglett.5c00433. Epub 2025 Mar 2. Org Lett. 2025. PMID: 40025849 Free PMC article.
-
Fluorogenic Trp(redBODIPY) cyclopeptide targeting keratin 1 for imaging of aggressive carcinomas.Chem Sci. 2019 Dec 18;11(5):1368-1374. doi: 10.1039/c9sc05558d. Chem Sci. 2019. PMID: 34123261 Free PMC article.
-
Ru(II)-Catalyzed Regiospecific C-H/O-H Oxidative Annulation to Access Isochromeno[8,1-ab]phenazines: Far-Red Fluorescence and Live Cancer Cell Imaging.ACS Omega. 2017 Jun 30;2(6):2694-2705. doi: 10.1021/acsomega.7b00335. Epub 2017 Jun 17. ACS Omega. 2017. PMID: 30023674 Free PMC article.
-
Synthesis and Photophysical Properties of Charge-Transfer-Based Pyrimidine-Derived α-Amino Acids.J Org Chem. 2023 Sep 15;88(18):13214-13224. doi: 10.1021/acs.joc.3c01437. Epub 2023 Aug 24. J Org Chem. 2023. PMID: 37621156 Free PMC article.
-
Determination of biogenic amines in alcoholic beverages using a novel fluorogenic compound as derivatizing reagent.RSC Adv. 2021 Jun 1;11(32):19541-19550. doi: 10.1039/d1ra01436f. eCollection 2021 May 27. RSC Adv. 2021. PMID: 35479236 Free PMC article.
References
-
- Beechem J. M., Brand L. Annu. Rev. Biochem. 1985;54:43. - PubMed
-
- Lang K., Chin J. W. Chem. Rev. 2014;114:4764. - PubMed
- Venkatraman P., Nguyen T. T., Sainlos M., Bilsel O., Chitta S., Imperiali B., Stern L. J. Nat. Chem. Biol. 2007;3:222. - PMC - PubMed
- Wang J., Xie J., Schultz P. G. J. Am. Chem. Soc. 2006;128:8738. - PubMed
- Stromgaard A., Jensen A. A., Stromgaard K. ChemBioChem. 2004;5:909. - PubMed
- Cohen B. E., McAnaney T. B., Park E. S., Jan Y. N., Boxer S. G., Jan L. Y. Science. 2002;296:1700. - PubMed
- Dougherty D. A. Curr. Opin. Chem. Biol. 2000;4:645. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

