The boron-boron triple bond? A thermodynamic and force field based interpretation of the N-heterocyclic carbene (NHC) stabilization procedure
- PMID: 29560205
- PMCID: PMC5811121
- DOI: 10.1039/c4sc02997f
The boron-boron triple bond? A thermodynamic and force field based interpretation of the N-heterocyclic carbene (NHC) stabilization procedure
Abstract
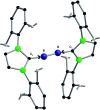
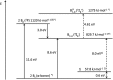
Recently, the NHC→B[triple bond, length as m-dash]B←NHC molecule 1 has been published in Science where it is described as a stabilized B2 molecule in its 1Σ excited state (B2*) (NHC = N-heterocyclic carbene C3N2H2(C6H3Pri 2-2,6)2). The bonding of 1 based on sophisticated calculations and the BB distances of the solid compound was discussed as the first example of a B2 triple bond in a stable molecule. Now we present an only experimentally based interpretation of 1 via detailed thermodynamic considerations, including its fragmentation to B2 molecules. Furthermore, from the vibrational spectrum force constants (f BB for the BB bond and f BC for the BC bond) were extracted, which are classical examples to indicate single, double and triple CC bonds in organic chemistry. The consequence of both properties of 1 (ΔE and f) generates a new interpretation which is in contrast to the triple bond donor-acceptor description visualized by arrows and which casts a critical light on the interpretation of any NHC "stabilized" molecule.
This journal is © The Royal Society of Chemistry 2015.
Figures

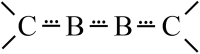
References
-
- Dalton Discussion 11: The Renaissance of Main Group Elements; Dalton Trans. 2008, 33, 4321–4524.. - PubMed
-
- Ecker A., Weckert E., Schnöckel H. Nature. 1997;387:379.
-
- Tran N. T., Powell D. R., Dahl L. F. Angew. Chem., Int. Ed. 2000;39:4121. - PubMed
-
- Jadzinsky P. D., Calero G., Ackerson C. J., Bushnell D. A., Kornberg R. D. Science. 2007;318:430. - PubMed
-
- Whetten R. L., Price R. C. Science. 2007;318:407. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

