Bioorthogonal prodrug activation driven by a strain-promoted 1,3-dipolar cycloaddition
- PMID: 29560207
- PMCID: PMC5811098
- DOI: 10.1039/c4sc02574a
Bioorthogonal prodrug activation driven by a strain-promoted 1,3-dipolar cycloaddition
Abstract
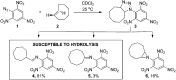
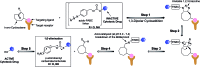
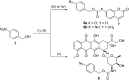
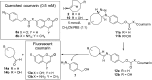
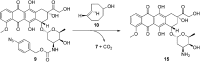
Due to the formation of hydrolysis-susceptible adducts, the 1,3-dipolar cycloaddition between an azide and strained trans-cyclooctene (TCO) has been disregarded in the field of bioorthogonal chemistry. We report a method which uses the instability of the adducts to our advantage in a prodrug activation strategy. The reaction of trans-cyclooctenol (TCO-OH) with a model prodrug resulted in a rapid 1,3-dipolar cycloaddition with second-order rates of 0.017 M-1 s-1 and 0.027 M-1 s-1 for the equatorial and axial isomers, respectively, resulting in release of the active compound. 1H NMR studies showed that activation proceeded via a triazoline and imine, both of which are rapidly hydrolyzed to release the model drug. Cytotoxicity of a doxorubicin prodrug was restored in vitro upon activation with TCO-OH, while with cis-cyclooctenol (CCO-OH) no activation was observed. The data also demonstrates the potential of this reaction in organic synthesis as a mild orthogonal protecting group strategy for amino and hydroxyl groups.
Figures


Similar articles
-
Mechanistic Evaluation of Bioorthogonal Decaging with trans-Cyclooctene: The Effect of Fluorine Substituents on Aryl Azide Reactivity and Decaging from the 1,2,3-Triazoline.Bioconjug Chem. 2018 Feb 21;29(2):324-334. doi: 10.1021/acs.bioconjchem.7b00665. Epub 2018 Jan 31. Bioconjug Chem. 2018. PMID: 29327914
-
Alkene-Azide 1,3-Dipolar Cycloaddition as a Trigger for Ultrashort Peptide Hydrogel Dissolution.Chem Asian J. 2019 Apr 15;14(8):1143-1150. doi: 10.1002/asia.201801184. Epub 2018 Nov 13. Chem Asian J. 2019. PMID: 30324726
-
EGFR-targeted prodrug activation using bioorthogonal alkene-azide click-and-release chemistry.Bioorg Med Chem. 2021 Sep 15;46:116361. doi: 10.1016/j.bmc.2021.116361. Epub 2021 Aug 8. Bioorg Med Chem. 2021. PMID: 34411983
-
IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications.Molecules. 2021 Jul 30;26(15):4640. doi: 10.3390/molecules26154640. Molecules. 2021. PMID: 34361793 Free PMC article. Review.
-
Electrophilic Azides for Materials Synthesis and Chemical Biology.Acc Chem Res. 2020 Apr 21;53(4):937-948. doi: 10.1021/acs.accounts.0c00046. Epub 2020 Mar 24. Acc Chem Res. 2020. PMID: 32207916 Review.
Cited by
-
Bioorthogonal Strategies for Engineering Extracellular Matrices.Adv Funct Mater. 2018 Mar 14;28(11):1706046. doi: 10.1002/adfm.201706046. Epub 2018 Jan 19. Adv Funct Mater. 2018. PMID: 31558890 Free PMC article.
-
Bioorthogonal chemistry.Nat Rev Methods Primers. 2021;1:30. doi: 10.1038/s43586-021-00028-z. Epub 2021 Apr 15. Nat Rev Methods Primers. 2021. PMID: 34585143 Free PMC article.
-
Unleashing the Power of Bond Cleavage Chemistry in Living Systems.ACS Cent Sci. 2021 Jun 23;7(6):929-943. doi: 10.1021/acscentsci.1c00124. Epub 2021 Apr 30. ACS Cent Sci. 2021. PMID: 34235254 Free PMC article. Review.
-
Click chemistry and drug delivery: A bird's-eye view.Acta Pharm Sin B. 2023 May;13(5):1990-2016. doi: 10.1016/j.apsb.2022.10.015. Epub 2022 Oct 22. Acta Pharm Sin B. 2023. PMID: 37250163 Free PMC article. Review.
-
Stable, Reactive, and Orthogonal Tetrazines: Dispersion Forces Promote the Cycloaddition with Isonitriles.Angew Chem Int Ed Engl. 2019 Jul 1;58(27):9043-9048. doi: 10.1002/anie.201903877. Epub 2019 Jun 6. Angew Chem Int Ed Engl. 2019. PMID: 31062496 Free PMC article.
References
-
- Collins I., Workman P. Nat. Chem. Biol. 2006;2:689–700. - PubMed
-
- Rautio J., Kumpulainen H., Heimbach T., Oliyai R., Oh D., Jarvinen T., Savolainen J. Nat. Rev. Drug Discovery. 2008;7:255–270. - PubMed
-
- Warnecke A., Drug Delivery in Oncology: From Basic Research to Cancer Therapy, ed. F. Kratz, P. Senter and H. Steinhagen, Wiley-VCH, Weinheim, Germany, 1st edn, 2011, pp. 553–589.
LinkOut - more resources
Full Text Sources
Other Literature Sources

