NMR and TRLFS studies of Ln(iii) and An(iii) C5-BPP complexes
- PMID: 29560242
- PMCID: PMC5811079
- DOI: 10.1039/c4sc03103b
NMR and TRLFS studies of Ln(iii) and An(iii) C5-BPP complexes
Abstract
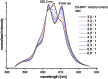
C5-BPP is a highly efficient N-donor ligand for the separation of trivalent actinides, An(iii), from trivalent lanthanides, Ln(iii). The molecular origin of the selectivity of C5-BPP and many other N-donor ligands of the BTP-type is still not entirely understood. We present here the first NMR studies on C5-BPP Ln(iii) and An(iii) complexes. C5-BPP is synthesized with 10% 15N labeling and characterized by NMR and LIFDI-MS methods. 15N NMR spectroscopy gives a detailed insight into the bonding of C5-BPP with lanthanides and Am(iii) as a representative for trivalent actinide cations, revealing significant differences in 15N chemical shift for coordinating nitrogen atoms compared to Ln(iii) complexes. The temperature dependence of NMR chemical shifts observed for the Am(iii) complex indicates a weak paramagnetism. This as well as the observed large chemical shift for coordinating nitrogen atoms show that metal-ligand bonding in Am(C5-BPP)3 has a larger share of covalence than in lanthanide complexes, confirming earlier studies. The Am(C5-BPP)3 NMR sample is furthermore spiked with Cm(iii) and characterized by time-resolved laser fluorescence spectroscopy (TRLFS), yielding important information on the speciation of trace amounts of minor complex species.
Figures






References
-
- IEA, Key World Energy Statistics 2012, International Energy Agency (IEA), Vienna, 2012.
-
- Gompper K., Geist A., Geckeis H. Nachr. Chem. 2010;58:1015–1019.
-
- Salvatores M., Palmiotti G. Prog. Part. Nucl. Phys. 2011;66:144–166.
-
- OECD Report NEA No. 6894 Potential Benefits and Impacts of Advanced Nuclear Fuel Cycles with Actinide Partitioning and Transmutation, OECD, Nuclear Energy Agency (NEA), Paris, 2011.
-
- Panak P. J., Geist A. Chem. Rev. 2013;113:1199–1236. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

