Utilization of gabapentin by people in treatment for substance use disorders in Belgium (2011-2014): a cross-sectional study
- PMID: 29560269
- PMCID: PMC5858131
- DOI: 10.1186/s13690-018-0254-8
Utilization of gabapentin by people in treatment for substance use disorders in Belgium (2011-2014): a cross-sectional study
Abstract
Background: Although gabapentin has been licensed in the European Union only for neuropathic pain and epilepsy for patients who have partial seizures, it has also been prescribed in treatment for substance use disorders. Many studies report the potential risk of abuse of gabapentin by people with substance use disorders. The objective of this paper is to determine if people who have been in treatment for substance use disorders bought gabapentin in a time span that could indicate consumption at a dose that exceeded the maximum approved dose of 3600 mg/day.
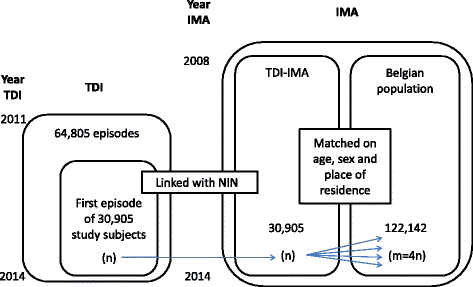
Methods: This analysis is the result of an observational cross-sectional descriptive study with matching. Two datasets were used and linked at individual level. Subjects were selected based on their first registration in the database of the Treatment Demand Indicator (TDI) between 2011 and 2014, without any exclusion criteria concerning nationality or age. Through linkage with the database of the InterMutualistic Agency (IMA) information on health service use and medication use was determined. In addition, each subject was matched on age, sex and place of residence to four comparators from the general population who were not in specialized treatment. The prevalence of gabapentin purchases in the period between 2008 and 2014 for both populations were compared. Quantification of the amount of gabapentin between two consecutive purchases was used as a proxy for potential abuse.
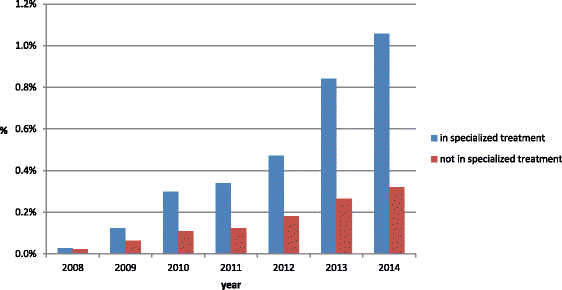
Results: Out of 30,905 patients in treatment for substance use disorders 2.7% had bought at least once gabapentin in a public pharmacy or received it from a hospital pharmacy, compared to 0.7% in the comparison group (n = 122,142). In both populations, more than half of the patients bought only once or twice gabapentin and about 10.0% bought at least once gabapentin in a time span that could indicate potential abuse. A limitation of the study is that it is only based on reimbursed medication without clinical information.
Conclusion: Through the linkage of the TDI-database and the database of the Belgian health insurance companies, no evidence was found for regular abuse of prescribed gabapentin in Belgium by people in treatment for substance use disorders.
Keywords: Belgium; Gabapentin; Health services; Pharmacoepidemiological data; Substance use disorders.
Conflict of interest statement
Not applicable.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- EMA - Committee for Medicinal Products for Human Use (CHMP). Referral opinion pursuant to article 30 of council directive 2001/83/EC for Neurontin and associated names. 4–8-2006.
LinkOut - more resources
Full Text Sources
Other Literature Sources