Micro RNAs are involved in activation of epicardium during zebrafish heart regeneration
- PMID: 29560280
- PMCID: PMC5849881
- DOI: 10.1038/s41420-018-0041-x
Micro RNAs are involved in activation of epicardium during zebrafish heart regeneration
Abstract
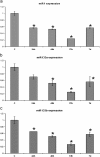
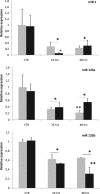
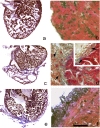
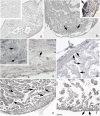
Zebrafish could be an interesting translational model to understand and improve the post-infarction trial and possible regeneration in humans. The adult zebrafish is able to regenerate efficiently after resecting nearly 20% of the ventricular apex. This process requires the concert activation of the epicardium and endocardium, as well as trans-differentiation of pre-existing cardiomyocytes that together replace the lost tissue. The molecular mechanisms involved in this activation process are not completely clarified. In this work, in order to investigate if the downregulation of these miRNAs (miRs) are linked with the activation of epicardium, the expressions of miR-133a, b and miR-1 during regeneration were analysed. qPCR analyses in whole-heart, or from distinct dissected epicardial cells comparing to regenerative clot (containing cardiomyocytes, fibroblasts and endocardial cells) by a laser-micro-dissector, have indicated that already at 24 h there is a downregulation of miRs: (1) miR-133a and miR-1 in the epicardium and (2) miR-133b and miR-1 in the regenerative clot. All the miRs remain downregulated until 7 days post-surgery. With the aim to visualize the activations of heart component in combination with miRs, we developed immunohistochemistry using antibodies directed against common markers in mammals as well as zebrafish: Wilms tumour 1 (WT1), a marker of epicardium; heat-shock protein 70 (HSP70), a chaperon activated during regeneration; and the Cardiac Troponin T (cTnT), a marker of differentiated cardiomyocytes. All these markers are directly or indirectly linked to the investigated miRs. WT1 and HSP70 strongly marked the regeneration site just at 2-3 days postventricular resection. In coherence, cTnT intensively marked the regenerative portion from 7 days onwards. miRs-1 and -133 (a,b) have been strongly involved in the activation of epicardium and regenerative clot during the regeneration process in zebrafish. This study can be a useful translational model to understand the early epicardial activation in which miRs-133a and miR-1 seem to play a central role as observed in the human heart.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Wu C, Weidinger G. Zebrafish as a model for studying cardiac regeneration. Curr. Pathobiol. Rep. 2014;2:93–100. doi: 10.1007/s40139-014-0042-2. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

