Neural mechanisms underlying successful and deficient multi-component behavior in early adolescent ADHD
- PMID: 29560310
- PMCID: PMC5857919
- DOI: 10.1016/j.nicl.2018.02.024
Neural mechanisms underlying successful and deficient multi-component behavior in early adolescent ADHD
Abstract
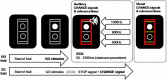
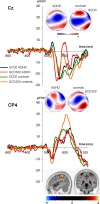
Attention Deficit Hyperactivity Disorder (ADHD) is a disorder affecting cognitive control. These functions are important to achieve goals when different actions need to be executed in close succession. This type of multi-component behavior, which often further requires the processing of information from different modalities, is important for everyday activities. Yet, possible changes in neurophysiological mechanisms have not been investigated in adolescent ADHD. We examined N = 31 adolescent ADHD patients and N = 35 healthy controls (HC) in two Stop-Change experiments using either uni-modal or bi-modal stimuli to trigger stop and change processes. These stimuli were either presented together (SCD0) or in close succession of 300 milliseconds (SCD300). Using event-related potentials (ERP), EEG data decomposition and source localization we analyzed neural processes and functional neuroanatomical correlates of multicomponent behavior. Compared to HCs, ADHD patients had longer reaction times and higher error rates when Stop and Change stimuli were presented in close succession (SCD300), but not when presented together (SCD0). This effect was evident in the uni-modal and bi-modal experiment and is reflected by neurophysiological processes reflecting response selection mechanisms in the inferior parietal cortex (BA40). These processes were only detectable after accounting for intra-individual variability in neurophysiological data; i.e. there were no effects in standard ERPs. Multi-component behavior is not always deficient in ADHD. Rather, modulations in multi-component behavior depend on a critical temporal integration window during response selection which is associated with functioning of the inferior parietal cortex. This window is smaller than in HCs and independent of the complexity of sensory input.
Keywords: ADHD; Cognitive control; Event related potential (ERP); Multi-component behavior, inferior parietal cortex; Neurophysiology.
Figures



References
-
- Bekker E.M., Overtoom C.C., Kenemans J.L., Kooij J.J., De Noord I., Buitelaar J.K., Verbaten M.N. Stopping and changing in adults with ADHD. Psychol. Med. 2005;35:807–816. - PubMed
-
- Beste C., Saft C. Action selection in a possible model of striatal medium spiny neuron dysfunction: behavioral and EEG data in a patient with benign hereditary chorea. Brain Struct. Funct. 2015;220:221–228. - PubMed
-
- Beste C., Stock A.-K., Epplen J.T., Arning L. On the relevance of the NPY2-receptor variation for modes of action cascading processes. NeuroImage. 2014;102(Pt 2):558–564. - PubMed
-
- Bluschke A., Roessner V., Beste C. Specific cognitive-neurophysiological processes predict impulsivity in the childhood attention-deficit/hyperactivity disorder combined subtype. Psychol. Med. 2016;46:1277–1287. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

