Cefepime Efficacy and Safety in Children: A Systematic Review and Meta-analysis
- PMID: 29560346
- PMCID: PMC5845692
- DOI: 10.3389/fped.2018.00046
Cefepime Efficacy and Safety in Children: A Systematic Review and Meta-analysis
Abstract
Background: Cefepime is a fourth-generation cephalosporin antibiotic used to treat a variety of infections. The US Food and Drug Administration approved its use in certain types of infections among pediatric patients, and yet there have been mixed data about its efficacy and safety in this population.
Objective: The objective of this review is to compare efficacy and all-cause mortality of cefepime to other clinically indicated antibiotics among children.
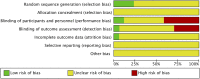
Methods: We conducted a systematic search of MEDLINE, EMBASE, CENTRAL, LILACS, and clinicaltrials.gov databases through February 8, 2016. We included randomized controlled trials comparing cefepime to other clinical antibiotics, placebo, or no treatment in children aged 0-19 years in the inpatient setting with clinical signs of infection. The primary outcome of interest was all-cause mortality. The secondary outcomes were success rate, treatment failure, and incidence of adverse events. Study quality was assessed using the Cochrane Risk of Bias Assessment Tool.
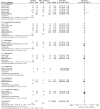
Results: Seventeen studies met the inclusion criteria. There was a total of 1,285 participants included, 624 participants in the cefepime arm and 661 in the comparison arm. A random effects meta-analysis for all-cause mortality showed no difference in rates of mortality between cefepime and comparator antibiotics with a mortality risk ratio of 0.88 (95% CI: 0.71-1.08). For the secondary outcomes of success rate and treatment failure, a random effects model meta-analysis conducted of the studies showed no difference in rate between cefepime and comparator antibiotics with an overall risk ratio of 0.98 (95% CI: 0.92-1.05) and 1.04 (95% CI: 0.91-1.19), respectively. Adverse events were not statistically assessed given widespread heterogeneity. Overall, the studies had unclear risk of bias and were limited by high heterogeneity and methodological flaws.
Conclusion: The efficacy and safety of cefepime in pediatric patients remain unclear despite the inclusion of newer trials since the last index systematic review conducted a decade ago.
Keywords: antibiotic; cefepime; cephalosporin; children; efficacy; safety.
Figures




Similar articles
-
Cochrane Review: Osmotic and stimulant laxatives for the management of childhood constipation (Review).Evid Based Child Health. 2013 Jan;8(1):57-109. doi: 10.1002/ebch.1893. Evid Based Child Health. 2013. PMID: 23878124 Review.
-
Antibiotics for secondary prevention of coronary heart disease.Cochrane Database Syst Rev. 2021 Feb 23;2(2):CD003610. doi: 10.1002/14651858.CD003610.pub4. Cochrane Database Syst Rev. 2021. PMID: 33704780 Free PMC article.
-
Interventions to reduce Staphylococcus aureus in the management of eczema.Cochrane Database Syst Rev. 2019 Oct 29;2019(10):CD003871. doi: 10.1002/14651858.CD003871.pub3. Cochrane Database Syst Rev. 2019. PMID: 31684694 Free PMC article.
-
Prophylactic antibiotics to prevent surgical site infection after breast cancer surgery.Cochrane Database Syst Rev. 2019 Sep 26;9(9):CD005360. doi: 10.1002/14651858.CD005360.pub5. Cochrane Database Syst Rev. 2019. PMID: 31557310 Free PMC article.
-
Antibiotics for induction and maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Feb 7;2(2):CD012730. doi: 10.1002/14651858.CD012730.pub2. Cochrane Database Syst Rev. 2019. PMID: 30731030 Free PMC article.
Cited by
-
Development and validation of a volumetric absorptive microsampling- liquid chromatography mass spectrometry method for the analysis of cefepime in human whole blood: Application to pediatric pharmacokinetic study.J Pharm Biomed Anal. 2020 Feb 5;179:113002. doi: 10.1016/j.jpba.2019.113002. Epub 2019 Nov 20. J Pharm Biomed Anal. 2020. PMID: 31785929 Free PMC article.
-
Phenotypic characterization and whole genome analysis of extended-spectrum beta-lactamase-producing bacteria isolated from dogs in Germany.PLoS One. 2018 Oct 26;13(10):e0206252. doi: 10.1371/journal.pone.0206252. eCollection 2018. PLoS One. 2018. PMID: 30365516 Free PMC article.
References
-
- AHFS Consumer Medication Information. Cefepime Injection. Bethesda, MD: American Society of Health-System Pharmacists, Inc. (2016). Available from: https://www.nlm.nih.gov/medlineplus/druginfo/meds/a698021.html
-
- Food and Drug Administration (FDA). Early Communication About an Ongoing Safety Review of Cefepime (Marketed as Maxipime). (2007). Available from: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforP...
-
- Food and Drug Administration (FDA). Cefepime (Marketed as Maxipime) Safety Review: An Update. (2009). Available from: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforP...
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

